Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease
- PMID: 22966038
- PMCID: PMC3462991
- DOI: 10.1124/pr.111.004846
Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease
Abstract
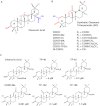
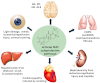
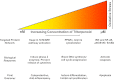
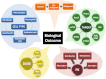
We review the rationale for the use of synthetic oleanane triterpenoids (SOs) for prevention and treatment of disease, as well as extensive biological data on this topic resulting from both cell culture and in vivo studies. Emphasis is placed on understanding mechanisms of action. SOs are noncytotoxic drugs with an excellent safety profile. Several hundred SOs have now been synthesized and in vitro have been shown to: 1) suppress inflammation and oxidative stress and therefore be cytoprotective, especially at low nanomolar doses, 2) induce differentiation, and 3) block cell proliferation and induce apoptosis at higher micromolar doses. Animal data on the use of SOs in neurodegenerative diseases and in diseases of the eye, lung, cardiovascular system, liver, gastrointestinal tract, and kidney, as well as in cancer and in metabolic and inflammatory/autoimmune disorders, are reviewed. The importance of the cytoprotective Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1/nuclear factor (erythroid-derived 2)-like 2/antioxidant response element (Keap1/Nrf2/ARE) pathway as a mechanism of action is explained, but interactions with peroxisome proliferator-activated receptor γ (PARPγ), inhibitor of nuclear factor-κB kinase complex (IKK), janus tyrosine kinase/signal transducer and activator of transcription (JAK/STAT), human epidermal growth factor receptor 2 (HER2)/ErbB2/neu, phosphatase and tensin homolog (PTEN), the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway, mammalian target of rapamycin (mTOR), and the thiol proteome are also described. In these interactions, Michael addition of SOs to reactive cysteine residues in specific molecular targets triggers biological activity. Ultimately, SOs are multifunctional drugs that regulate the activity of entire networks. Recent progress in the earliest clinical trials with 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) methyl ester (bardoxolone methyl) is also summarized.
Figures
References
-
- Abel EL, Bubel JD, Simper MS, Powell L, McClellan SA, Andreeff M, MacLeod MC, DiGiovanni J. (2011) Protection against 2-chloroethyl ethyl sulfide (CEES)-induced cytotoxicity in human keratinocytes by an inducer of the glutathione detoxification pathway. Toxicol Appl Pharmacol 255:176–183 - PubMed
-
- Ahmad R, Liu S, Weisberg E, Nelson E, Galinsky I, Meyer C, Kufe D, Kharbanda S, Stone R. (2010) Combining the FLT3 inhibitor PKC412 and the triterpenoid CDDO-Me synergistically induces apoptosis in acute myeloid leukemia with the internal tandem duplication mutation. Mol Cancer Res 8:986–993 - PMC - PubMed
-
- Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. (2006) Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem 281:35764–35769 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

