Enteropathogenic Escherichia coli and vaccinia virus do not require the family of WASP-interacting proteins for pathogen-induced actin assembly
- PMID: 22966049
- PMCID: PMC3497443
- DOI: 10.1128/IAI.06148-11
Enteropathogenic Escherichia coli and vaccinia virus do not require the family of WASP-interacting proteins for pathogen-induced actin assembly
Abstract
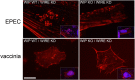
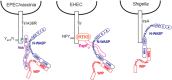
The human pathogens enteropathogenic Escherichia coli (EPEC) and vaccinia virus trigger actin assembly in host cells by activating the host adaptor Nck and the actin nucleation promoter neural Wiskott-Aldrich syndrome protein (N-WASP). EPEC translocates effector molecules into host cells via type III secretion, and the interaction between the translocated intimin receptor (Tir) and the bacterial membrane protein intimin stimulates Nck and N-WASP recruitment, leading to the formation of actin pedestals beneath adherent bacteria. Vaccinia virus also recruits Nck and N-WASP to generate actin tails that promote cell-to-cell spread of the virus. In addition to Nck and N-WASP, WASP-interacting protein (WIP) localizes to vaccinia virus tails, and inhibition of actin tail formation upon ectopic expression of WIP mutants led to the suggestion that WIP is required for this process. Similar studies of WIP mutants, however, did not affect the ability of EPEC to form actin pedestals, arguing against an essential role for WIP in EPEC-induced actin assembly. In this study, we demonstrate that Nck and N-WASP are normally recruited by vaccinia virus and EPEC in the absence of WIP, and neither WIP nor the WIP family members CR16 and WIRE/WICH are essential for pathogen induced actin assembly. In addition, although Nck binds EPEC Tir directly, N-WASP is required for its localization during pedestal formation. Overall, these data highlight similar pathogenic strategies shared by EPEC and vaccinia virus by demonstrating a requirement for both Nck and N-WASP, but not WIP or WIP family members in pathogen-induced actin assembly.
Figures





References
-
- Anton I, et al. 2003. WIP participates in actin reorganization and ruffle formation induced by PDGF. J. Cell Sci. 116:2443–2451 - PubMed
-
- Anton IM, et al. 2002. WIP deficiency reveals a differential role for WIP and the actin cytoskeleton in T and B cell activation. Immunity 16:193–204 - PubMed
-
- Anton IM, Jones GE. 2006. WIP: a multifunctional protein involved in actin cytoskeleton regulation. Eur. J. Cell Biol. 85(3–4):295–304 - PubMed
-
- Anton IM, Lu W, Mayer BJ, Ramesh N, Geha RS. 1998. The Wiskott-Aldrich syndrome protein-interacting protein (WIP) binds to the adaptor protein Nck. J. Biol. Chem. 273:20992–20995 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 DK088442/DK/NIDDK NIH HHS/United States
- R01AI052354/AI/NIAID NIH HHS/United States
- K08 DK094966/DK/NIDDK NIH HHS/United States
- 5P30DK034854/DK/NIDDK NIH HHS/United States
- R01 AI046454/AI/NIAID NIH HHS/United States
- R01 AI052354/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01AI46454/AI/NIAID NIH HHS/United States
- F32DK088442/DK/NIDDK NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
- P01HL059561/HL/NHLBI NIH HHS/United States
- P01 HL059561/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

