Antigenic characterization of an intrinsically unstructured protein, Plasmodium falciparum merozoite surface protein 2
- PMID: 22966050
- PMCID: PMC3497424
- DOI: 10.1128/IAI.00665-12
Antigenic characterization of an intrinsically unstructured protein, Plasmodium falciparum merozoite surface protein 2
Abstract
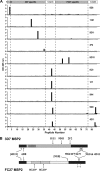
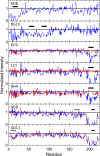
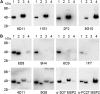
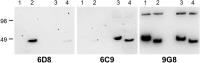
Merozoite surface protein 2 (MSP2) is an abundant glycosylphosphatidylinositol (GPI)-anchored protein of Plasmodium falciparum, which is a potential component of a malaria vaccine. As all forms of MSP2 can be categorized into two allelic families, a vaccine containing two representative forms of MSP2 may overcome the problem of diversity in this highly polymorphic protein. Monomeric recombinant MSP2 is an intrinsically unstructured protein, but its conformational properties on the merozoite surface are unknown. This question is addressed here by analyzing the 3D7 and FC27 forms of recombinant and parasite MSP2 using a panel of monoclonal antibodies raised against recombinant MSP2. The epitopes of all antibodies, mapped using both a peptide array and by nuclear magnetic resonance (NMR) spectroscopy on full-length recombinant MSP2, were shown to be linear. The antibodies revealed antigenic differences, which indicate that the conserved N- and C-terminal regions, but not the central variable region, are less accessible in the parasite antigen. This appears to be an intrinsic property of parasite MSP2 and is not dependent on interactions with other merozoite surface proteins as the loss of some conserved-region epitopes seen using the immunofluorescence assay (IFA) on parasite smears was also seen on Western blot analyses of parasite lysates. Further studies of the structural basis of these antigenic differences are required in order to optimize recombinant MSP2 constructs being evaluated as potential vaccine components.
Figures
Similar articles
-
Structure and Characterisation of a Key Epitope in the Conserved C-Terminal Domain of the Malaria Vaccine Candidate MSP2.J Mol Biol. 2017 Mar 24;429(6):836-846. doi: 10.1016/j.jmb.2017.02.003. Epub 2017 Feb 8. J Mol Biol. 2017. PMID: 28189425
-
Conformational dynamics and antigenicity in the disordered malaria antigen merozoite surface protein 2.PLoS One. 2015 Mar 5;10(3):e0119899. doi: 10.1371/journal.pone.0119899. eCollection 2015. PLoS One. 2015. PMID: 25742002 Free PMC article.
-
Lipid interactions modulate the structural and antigenic properties of the C-terminal domain of the malaria antigen merozoite surface protein 2.FEBS J. 2017 Aug;284(16):2649-2662. doi: 10.1111/febs.14135. Epub 2017 Jul 5. FEBS J. 2017. PMID: 28618199
-
Plasmodium falciparum merozoite surface protein 2 is unstructured and forms amyloid-like fibrils.Mol Biochem Parasitol. 2009 Aug;166(2):159-71. doi: 10.1016/j.molbiopara.2009.03.012. Epub 2009 Apr 9. Mol Biochem Parasitol. 2009. PMID: 19450733 Free PMC article.
-
Recombinant protein vaccines against the asexual blood stages of Plasmodium falciparum.Hum Vaccin. 2010 Jan;6(1):39-53. doi: 10.4161/hv.6.1.10712. Epub 2010 Jan 19. Hum Vaccin. 2010. PMID: 20061790 Review.
Cited by
-
Improving serodiagnosis of human and canine leishmaniasis with recombinant Leishmania braziliensis cathepsin l-like protein and a synthetic peptide containing its linear B-cell epitope.PLoS Negl Trop Dis. 2015 Jan 8;9(1):e3426. doi: 10.1371/journal.pntd.0003426. eCollection 2015 Jan. PLoS Negl Trop Dis. 2015. PMID: 25569432 Free PMC article.
-
Apicoplast-derived isoprenoids are essential for biosynthesis of GPI protein anchors, and consequently for egress and invasion in Plasmodium falciparum.PLoS Pathog. 2024 Sep 6;20(9):e1012484. doi: 10.1371/journal.ppat.1012484. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39241090 Free PMC article.
-
A genomic platform for surveillance and antigen discovery in Plasmodium spp. using long-read amplicon sequencing.Cell Rep Methods. 2023 Sep 25;3(9):100574. doi: 10.1016/j.crmeth.2023.100574. Epub 2023 Aug 29. Cell Rep Methods. 2023. PMID: 37751696 Free PMC article.
-
Sequential processing of merozoite surface proteins during and after erythrocyte invasion by Plasmodium falciparum.Infect Immun. 2014 Mar;82(3):924-36. doi: 10.1128/IAI.00866-13. Epub 2013 Nov 11. Infect Immun. 2014. PMID: 24218484 Free PMC article.
-
Naturally acquired IgG responses to Plasmodium falciparum do not target the conserved termini of the malaria vaccine candidate Merozoite Surface Protein 2.Front Immunol. 2024 Dec 9;15:1501700. doi: 10.3389/fimmu.2024.1501700. eCollection 2024. Front Immunol. 2024. PMID: 39717775 Free PMC article.
References
-
- Anders RF, et al. 1998. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine 16:240–247 - PubMed
-
- Angov E, et al. 2003. Development and pre-clinical analysis of a Plasmodium falciparum merozoite surface protein-1(42) malaria vaccine. Mol. Biochem. Parasitol. 128:195–204 - PubMed
-
- Barry AE, Schultz L, Buckee CO, Reeder JC. 2009. Contrasting population structures of the genes encoding ten leading vaccine-candidate antigens of the human malaria parasite, Plasmodium falciparum. PLoS One 4:e8497 doi:10.1371/journal.pone.0008497 - DOI - PMC - PubMed
-
- Blackman MJ, Ling IT, Nicholls SC, Holder AA. 1991. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol. Biochem. Parasitol. 49:29–33 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

