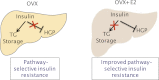
Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance
- PMID: 22966069
- PMCID: PMC3554377
- DOI: 10.2337/db11-1718
Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance
Abstract
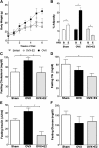
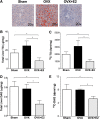
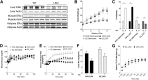
Pathway-selective insulin resistance where insulin fails to suppress hepatic glucose production but promotes liver fat storage may underlie glucose and lipid abnormalities after menopause. We tested the mechanisms by which estrogen treatment may alter the impact of a high-fat diet (HFD) when given at the time of ovariectomy (OVX) in mice. Female C57BL/6J mice underwent sham operation, OVX, or OVX with estradiol (E2) treatment and were fed an HFD. Hyperinsulinemic-euglycemic clamps were used to assess insulin sensitivity, tracer incorporation into hepatic lipids, and liver triglyceride export. OVX mice had increased adiposity that was prevented with E2 at the time of OVX. E2 treatment increased insulin sensitivity with OVX and HFD. In sham and OVX mice, HFD feeding induced fatty liver, and insulin reduced hepatic apoB100 and liver triglyceride export. E2 treatment reduced liver lipid deposition and prevented the decrease in liver triglyceride export during hyperinsulinemia. In mice lacking the liver estrogen receptor α, E2 after OVX limited adiposity but failed to improve insulin sensitivity, to limit liver lipid deposition, and to prevent insulin suppression of liver triglyceride export. In conclusion, estrogen treatment may reverse aspects of pathway-selective insulin resistance by promoting insulin action on glucose metabolism but limiting hepatic lipid deposition.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

