Tub has a key role in insulin and leptin signaling and action in vivo in hypothalamic nuclei
- PMID: 22966070
- PMCID: PMC3526052
- DOI: 10.2337/db11-1388
Tub has a key role in insulin and leptin signaling and action in vivo in hypothalamic nuclei
Retraction in
-
Statement of Retraction. Tub Has a Key Role in Insulin and Leptin Signaling and Action In Vivo in Hypothalamic Nuclei. Diabetes 2013;62:137-148. DOI: 10.2337/db11-1388.Diabetes. 2017 Mar;66(3):785-786. doi: 10.2337/db17-rt03a. Epub 2017 Feb 10. Diabetes. 2017. PMID: 28188142 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern. Tub Has a Key Role in Insulin and Leptin Signaling and Action In Vivo in Hypothalamic Nuclei. Diabetes 2013;62:137-148. DOI: 10.2337/db11-1388.Diabetes. 2016 Apr;65(4):1121-2. doi: 10.2337/db16-ec04. Diabetes. 2016. PMID: 27208021 Free PMC article. No abstract available.
Abstract
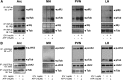
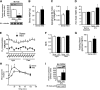
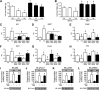
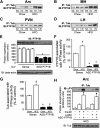
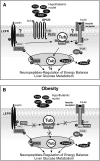
Mutation of tub gene in mice induces obesity, suggesting that tub could be an important regulator of energy balance. In the current study, we investigated whether insulin, leptin, and obesity can modulate Tub in vivo in hypothalamic nuclei, and we investigated possible consequences on energy balance, neuropeptide expression, and hepatic glucose metabolism. Food intake, metabolic characteristics, signaling proteins, and neuropeptide expression were measured in response to fasting and refeeding, intracerebroventricular insulin and leptin, and Tub antisense oligonucleotide (ASO). Tub tyrosine phosphorylation (Tub-p-tyr) is modulated by nutritional status. Tub is a substrate of insulin receptor tyrosine kinase (IRTK) and leptin receptor (LEPR)-Janus kinase 2 (JAK2) in hypothalamic nuclei. After leptin or insulin stimulation, Tub translocates to the nucleus. Inhibition of Tub expression in hypothalamus by ASO increased food intake, fasting blood glucose, and hepatic glucose output, decreased O(2) consumption, and blunted the effect of insulin or leptin on proopiomelanocortin, thyroid-releasing hormone, melanin-concentrating hormone, and orexin expression. In hypothalamus of mice administered a high-fat diet, there is a reduction in leptin and insulin-induced Tub-p-tyr and nuclear translocation, which is reversed by reducing protein tyrosine phosphatase 1B expression. These results indicate that Tub has a key role in the control of insulin and leptin effects on food intake, and the modulation of Tub may contribute to insulin and leptin resistance in DIO mice.
Figures



References
-
- Belgardt BF, Brüning JC. CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci 2010;1212:97–113 - PubMed
-
- Carvalheira JB, Torsoni MA, Ueno M, et al. Cross-talk between the insulin and leptin signaling systems in rat hypothalamus. Obes Res 2005;13:48–57 - PubMed
-
- Morton GJ, Schwartz MW. The NPY/AgRP neuron and energy homeostasis. Int J Obes Relat Metab Disord 2001;25(Suppl 5):S56–S62 - PubMed
-
- Woods SC, Schwartz MW, Baskin DG, Seeley RJ. Food intake and the regulation of body weight. Annu Rev Psychol 2000;51:255–277 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

