The Wnt signaling pathway effector TCF7L2 controls gut and brain proglucagon gene expression and glucose homeostasis
- PMID: 22966074
- PMCID: PMC3581223
- DOI: 10.2337/db12-0365
The Wnt signaling pathway effector TCF7L2 controls gut and brain proglucagon gene expression and glucose homeostasis
Abstract
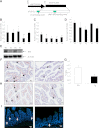
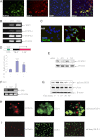
The type 2 diabetes risk gene TCF7L2 is the effector of the Wnt signaling pathway. We found previously that in gut endocrine L-cell lines, TCF7L2 controls transcription of the proglucagon gene (gcg), which encodes the incretin hormone glucagon-like peptide-1 (GLP-1). Whereas peripheral GLP-1 stimulates insulin secretion, brain GLP-1 controls energy homeostasis through yet-to-be defined mechanisms. We aim to determine the metabolic effect of a functional knockdown of TCF7L2 by generating transgenic mice that express dominant-negative TCF7L2 (TCF7L2DN) specifically in gcg-expressing cells. The gcg-TCF7L2DN transgenic mice showed reduced gcg expression in their gut and brain, but not in pancreas. Defects in glucose homeostasis were observed in these mice, associated with attenuated plasma insulin levels in response to glucose challenge. The defect in glucose disposal was exacerbated with high-fat diet. Brain Wnt activity and feeding-mediated hypothalamic AMP-activated protein kinase (AMPK) repression in these mice were impaired. Peripheral injection of the cAMP-promoting agent forskolin increased brain β-cat Ser675 phosphorylation and brain gcg expression and restored feeding-mediated hypothalamic AMPK repression. We conclude that TCF7L2 and Wnt signaling control gut and brain gcg expression and glucose homeostasis and speculate that positive cross-talk between Wnt and GLP-1/cAMP signaling is an underlying mechanism for brain GLP-1 in exerting its metabolic functions.
Figures




Comment in
-
TCF7L2 and glucose metabolism: time to look beyond the pancreas.Diabetes. 2013 Mar;62(3):706-8. doi: 10.2337/db12-1418. Diabetes. 2013. PMID: 23431017 Free PMC article. No abstract available.
References
-
- Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 2006;38:320–323 - PubMed
-
- Osmark P, Hansson O, Jonsson A, Rönn T, Groop L, Renström E. Unique splicing pattern of the TCF7L2 gene in human pancreatic islets. Diabetologia 2009;52:850–854 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

