The effects of slow skeletal troponin I expression in the murine myocardium are influenced by development-related shifts in myosin heavy chain isoform
- PMID: 22966157
- PMCID: PMC3530116
- DOI: 10.1113/jphysiol.2012.240085
The effects of slow skeletal troponin I expression in the murine myocardium are influenced by development-related shifts in myosin heavy chain isoform
Abstract
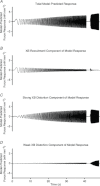
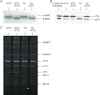
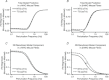
Troponin I (TnI) and myosin heavy chain (MHC) are two contractile regulatory proteins that undergo major shifts in isoform expression as cardiac myocytes mature from embryonic to adult stages. To date, many studies have investigated individual effects of embryonic vs. cardiac isoforms of either TnI or MHC on cardiac muscle function and contractile dynamics. Thus, we sought to determine whether concomitant expression of the embryonic isoforms of both TnI and MHC had functional effects that were not previously observed. Adult transgenic (TG) mice that express the embryonic isoform of TnI, slow skeletal TnI (ssTnI), were treated with propylthiouracil (PTU) to revert MHC expression from adult (α-MHC) to embryonic (β-MHC) isoforms. Cardiac muscle fibres from these mice contained ∼80% β-MHC and ∼34% ssTnI of total MHC or TnI, respectively, allowing us to test the functional effects of ssTnI in the presence of β-MHC. Detergent-skinned cardiac muscle fibre bundles were used to study how the interplay between MHC and TnI modulate muscle length-mediated effect on crossbridge (XB) recruitment dynamics, Ca(2+)-activated tension, and ATPase activity. One major finding was that the model-predicted XB recruitment rate (b) was enhanced significantly by ssTnI, and this speeding effect of ssTnI on XB recruitment rate was much greater (3.8-fold) when β-MHC was present. Another major finding was that the previously documented ssTnI-mediated increase in myofilament Ca(2+) sensitivity (pCa(50)) was blunted when β-MHC was present. ssTnI expression increased pCa(50) by 0.33 in α-MHC fibres, whereas ssTnI increased pCa(50) by only 0.05 in β-MHC fibres. Our study provides new evidence for significant interplay between MHC and TnI isoforms that is essential for tuning cardiac contractile function. Thus, MHC-TnI interplay may provide a developmentally dependent mechanism to enhance XB recruitment dynamics at a time when Ca(2+)-handling mechanisms are underdeveloped, and to prevent excessive ssTnI-dependent inotropy (increased Ca(2+) sensitivity) in the embryonic myocardium.
Figures



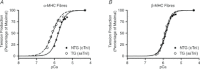
Similar articles
-
Expression of slow skeletal troponin I in adult transgenic mouse heart muscle reduces the force decline observed during acidic conditions.J Physiol. 2001 Nov 1;536(Pt 3):863-70. doi: 10.1111/j.1469-7793.2001.00863.x. J Physiol. 2001. PMID: 11691878 Free PMC article.
-
Effects of R92 mutations in mouse cardiac troponin T are influenced by changes in myosin heavy chain isoform.J Mol Cell Cardiol. 2012 Oct;53(4):542-51. doi: 10.1016/j.yjmcc.2012.07.018. Epub 2012 Aug 4. J Mol Cell Cardiol. 2012. PMID: 22884844 Free PMC article.
-
Chimera analysis of troponin I domains that influence Ca(2+)-activated myofilament tension in adult cardiac myocytes.Circ Res. 2000 Mar 3;86(4):470-7. doi: 10.1161/01.res.86.4.470. Circ Res. 2000. PMID: 10700453
-
Developmental changes in contractility and sarcomeric proteins from the early embryonic to the adult stage in the mouse heart.J Physiol. 2003 Apr 15;548(Pt 2):493-505. doi: 10.1113/jphysiol.2002.036509. Epub 2003 Mar 14. J Physiol. 2003. PMID: 12640016 Free PMC article.
-
The miscommunicative cardiac cell: when good proteins go bad.Ann N Y Acad Sci. 2005 Jun;1047:30-7. doi: 10.1196/annals.1341.003. Ann N Y Acad Sci. 2005. PMID: 16093482 Review.
Cited by
-
Deletion of Enigma Homologue from the Z-disc slows tension development kinetics in mouse myocardium.J Gen Physiol. 2019 May 6;151(5):670-679. doi: 10.1085/jgp.201812214. Epub 2019 Jan 14. J Gen Physiol. 2019. PMID: 30642915 Free PMC article.
-
Comparison of elementary steps of the cross-bridge cycle in rat papillary muscle fibers expressing α- and β-myosin heavy chain with sinusoidal analysis.J Muscle Res Cell Motil. 2016 Dec;37(6):203-214. doi: 10.1007/s10974-016-9456-2. Epub 2016 Dec 10. J Muscle Res Cell Motil. 2016. PMID: 27942960
-
Altered Right Ventricular Mechanical Properties Are Afterload Dependent in a Rodent Model of Bronchopulmonary Dysplasia.Front Physiol. 2017 Oct 25;8:840. doi: 10.3389/fphys.2017.00840. eCollection 2017. Front Physiol. 2017. PMID: 29118720 Free PMC article.
-
Time-regulated transcripts with the potential to modulate human pluripotent stem cell-derived cardiomyocyte differentiation.Stem Cell Res Ther. 2022 Sep 2;13(1):437. doi: 10.1186/s13287-022-03138-x. Stem Cell Res Ther. 2022. PMID: 36056380 Free PMC article.
-
Length-dependent effects on cardiac contractile dynamics are different in cardiac muscle containing α- or β-myosin heavy chain.Arch Biochem Biophys. 2013 Jul 1;535(1):3-13. doi: 10.1016/j.abb.2012.10.011. Epub 2012 Oct 27. Arch Biochem Biophys. 2013. PMID: 23111184 Free PMC article.
References
-
- Arteaga GM, Warren CM, Milutinovic S, Martin AF, Solaro RJ. Specific enhancement of sarcomeric response to Ca2+ protects murine myocardium against ischemia-reperfusion dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H2183–2192. - PubMed
-
- Baker AJ, Figueredo VM, Keung EC, Camacho SA. Ca2+ regulates the kinetics of tension development in intact cardiac muscle. Am J Physiol Heart Circ Physiol. 1998;275:H744–750. - PubMed
-
- Bhavsar PK, Dhoot GK, Cumming DV, Butler-Browne GS, Yacoub MH, Barton PJ. Developmental expression of troponin I isoforms in fetal human heart. FEBS Lett. 1991;292:5–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

