PIP2 hydrolysis stimulates the electrogenic Na+-bicarbonate cotransporter NBCe1-B and -C variants expressed in Xenopus laevis oocytes
- PMID: 22966160
- PMCID: PMC3530112
- DOI: 10.1113/jphysiol.2012.242479
PIP2 hydrolysis stimulates the electrogenic Na+-bicarbonate cotransporter NBCe1-B and -C variants expressed in Xenopus laevis oocytes
Abstract
Electrogenic Na(+)-bicarbonate cotransporter NBCe1 variants contribute to pH(i) regulation, and promote ion reabsorption or secretion by many epithelia. Most Na(+)-coupled bicarbonate transporter (NCBT) families such as NBCe1 contain variants with differences primarily at the cytosolic N and/or C termini that are likely to impart on the transporters different modes of regulation. For example, N-terminal regions of NBCe1 autoregulate activity. Our group previously reported that cytosolic phosphatidylinositol 4,5-bisphosphate (PIP(2)) stimulates heterologously expressed rat NBCe1-A in inside-out macropatches excised from Xenopus laevis oocytes. In the current study on whole oocytes, we used the two-electrode voltage-clamp technique, as well as pH- and voltage-sensitive microelectrodes, to characterize the effect of injecting PIP(2) on the activity of heterologously expressed NBCe1-A, -B, or -C. Injecting PIP(2) (10 μM estimated final) into voltage-clamped oocytes stimulated NBC-mediated, HCO(3)(-)-induced outward currents by >100% for the B and C variants, but not for the A variant. The majority of this stimulation involved PIP(2) hydrolysis and endoplasmic reticulum (ER) Ca(2+) release. Stimulation by PIP(2) injection was mimicked by injecting IP(3), but inhibited by either applying the phospholipase C (PLC) inhibitor U73112 or depleting ER Ca(2+) with prolonged thapsigargin/EGTA treatment. Stimulating the activity of store-operated Ca(2+) channels (SOCCs) to trigger a Ca(2+) influx mimicked the PIP(2)/IP(3) stimulation of the B and C variants. Activating the endogenous G(q) protein-coupled receptor in oocytes with lysophosphatidic acid (LPA) also stimulated the B and C variants in a Ca(2+)-dependent manner, although via an increase in surface expression for the B variant. In simultaneous voltage-clamp and pH(i) studies on NBCe1-C-expressing oocytes, LPA increased the NBC-mediated pH(i)-recovery rate from a CO(2)-induced acid load by ∼80%. Finally, the general kinase inhibitor staurosporine completely inhibited the IP(3)-induced stimulation of NBCe1-C. In summary, injecting PIP(2) stimulates the activity of NBCe1-B and -C expressed in oocytes through an increase in IP(3)/Ca(2+) that involves a staurosporine-sensitive kinase. In conjunction with our previous macropatch findings, PIP(2) regulates NBCe1 through a dual pathway involving both a direct stimulatory effect of PIP(2) on at least NBCe1-A, as well as an indirect stimulatory effect of IP(3)/Ca(2+) on the B and C variants.
Figures



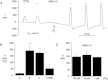



 stimulated the HCO3−-induced outward currents by ∼3-fold compared to the currents in the absence of
stimulated the HCO3−-induced outward currents by ∼3-fold compared to the currents in the absence of  . These stimulated currents were reversed by removing
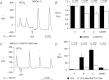
. These stimulated currents were reversed by removing  . B, summary data of the mean percentage NBC stimulation from panel A-type experiments on the three variants, as well as CΔN87. ***P ≤ 0.001 and **P ≤ 0.01 for the mean current pre- vs. post-injection. n ≥ 3 for each bar. C, summary of mean single-oocyte chemiluminescence (SOC) from oocytes first pretreated with the 0 Ca2+/EGTA/TG solution, and then either mainta-ined in 0 Ca2+ (filled bars) or re-exposed to
. B, summary data of the mean percentage NBC stimulation from panel A-type experiments on the three variants, as well as CΔN87. ***P ≤ 0.001 and **P ≤ 0.01 for the mean current pre- vs. post-injection. n ≥ 3 for each bar. C, summary of mean single-oocyte chemiluminescence (SOC) from oocytes first pretreated with the 0 Ca2+/EGTA/TG solution, and then either mainta-ined in 0 Ca2+ (filled bars) or re-exposed to  for 5 min (open bars). n ≥ 20 (2 oocyte batches) for each bar.
for 5 min (open bars). n ≥ 20 (2 oocyte batches) for each bar.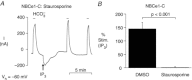

Similar articles
-
Phosphatidylinositol 4,5-bisphosphate degradation inhibits the Na+/bicarbonate cotransporter NBCe1-B and -C variants expressed in Xenopus oocytes.J Physiol. 2015 Feb 1;593(3):541-58. doi: 10.1113/jphysiol.2014.284307. J Physiol. 2015. PMID: 25398525 Free PMC article.
-
Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini.J Gen Physiol. 2006 Jun;127(6):639-58. doi: 10.1085/jgp.200609520. J Gen Physiol. 2006. PMID: 16735752 Free PMC article.
-
Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates the electrogenic Na/HCO3 cotransporter NBCe1-A expressed in Xenopus oocytes.Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):14150-5. doi: 10.1073/pnas.0906303106. Epub 2009 Aug 10. Proc Natl Acad Sci U S A. 2009. PMID: 19667194 Free PMC article.
-
[Physiology and pathophysiology of Na⁺/HCO₃⁻ cotransporter NBCe1].Sheng Li Xue Bao. 2012 Dec 25;64(6):729-40. Sheng Li Xue Bao. 2012. PMID: 23258339 Review. Chinese.
-
NBCe1 as a model carrier for understanding the structure-function properties of Na⁺ -coupled SLC4 transporters in health and disease.Pflugers Arch. 2014 Aug;466(8):1501-16. doi: 10.1007/s00424-014-1448-8. Epub 2014 Feb 11. Pflugers Arch. 2014. PMID: 24515290 Free PMC article. Review.
Cited by
-
Comparison between drug-induced and K⁺-induced changes in molar acid extrusion fluxes (JH⁺) and in energy consumption rates in astrocytes.Neurochem Res. 2013 Nov;38(11):2364-74. doi: 10.1007/s11064-013-1149-2. Epub 2013 Sep 14. Neurochem Res. 2013. PMID: 24037520
-
Astrocytes regulate brain extracellular pH via a neuronal activity-dependent bicarbonate shuttle.Nat Commun. 2020 Oct 8;11(1):5073. doi: 10.1038/s41467-020-18756-3. Nat Commun. 2020. PMID: 33033238 Free PMC article.
-
Structural and functional insights into the lipid regulation of human anion exchanger 2.Nat Commun. 2024 Jan 26;15(1):759. doi: 10.1038/s41467-024-44966-0. Nat Commun. 2024. PMID: 38272905 Free PMC article.
-
Structural insights into the conformational changes of BTR1/SLC4A11 in complex with PIP2.Nat Commun. 2023 Oct 3;14(1):6157. doi: 10.1038/s41467-023-41924-0. Nat Commun. 2023. PMID: 37788993 Free PMC article.
-
The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters.Physiol Rev. 2013 Apr;93(2):803-959. doi: 10.1152/physrev.00023.2012. Physiol Rev. 2013. PMID: 23589833 Free PMC article. Review.
References
-
- Abuladze N, Song M, Pushkin A, Newman D, Lee I, Nicholas S, Kurtz I. Structural organization of the human NBC1 gene: kNBC1 is transcribed from an alternative promoter in intron 3. Gene. 2000;251:109–122. - PubMed
-
- Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol Cell. 2006;22:795–806. - PubMed
-
- Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na+-HCO3− cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. Am J Physiol Cell Physiol. 2000;278:C1200–C1211. - PubMed
-
- Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

