Platelets increase the proliferation of ovarian cancer cells
- PMID: 22966171
- PMCID: PMC3520623
- DOI: 10.1182/blood-2012-06-438598
Platelets increase the proliferation of ovarian cancer cells
Abstract
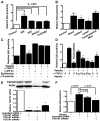
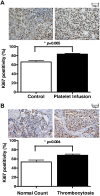
Platelets promote metastasis and angiogenesis, but their effect on tumor cell growth is uncertain. Here we report a direct proliferative effect of platelets on cancer cells both in vitro and in vivo. Incubation of platelets with ovarian cancer cells from murine (ID8 and 2C6) or human (SKOV3 and OVCAR5) origin increased cell proliferation. The proliferative effect of platelets was not dependent on direct contact with cancer cells, and preincubation of platelets with blocking antibodies against platelet adhesion molecules did not alter their effect on cancer cells. The proliferative effect of platelets was reduced by fixing platelets with paraformaldehyde, preincubating platelets with a TGF-β1-blocking antibody, or reducing expression of TGF-βR1 receptor on cancer cells with siRNA. Infusing platelets into mice with orthotopic ovarian tumors significantly increased the proliferation indices in these tumors. Ovarian cancer patients with thrombocytosis had higher tumor proliferation indices compared with patients with normal platelet counts.
Figures
Comment in
-
Platelets signal and tumors take off.Blood. 2012 Dec 6;120(24):4667-8. doi: 10.1182/blood-2012-09-457325. Blood. 2012. PMID: 23223212 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

