An Information Theory Approach to Nonlinear, Nonequilibrium Thermodynamics
- PMID: 22966210
- PMCID: PMC3436205
- DOI: 10.1007/s10955-011-0358-9
An Information Theory Approach to Nonlinear, Nonequilibrium Thermodynamics
Abstract
Using the problem of ion channel thermodynamics as an example, we illustrate the idea of building up complex thermodynamic models by successively adding physical information. We present a new formulation of information algebra that generalizes methods of both information theory and statistical mechanics. From this foundation we derive a theory for ion channel kinetics, identifying a nonequilibrium 'process' free energy functional in addition to the well-known integrated work functionals. The Gibbs-Maxwell relation for the free energy functional is a Green-Kubo relation, applicable arbitrarily far from equilibrium, that captures the effect of non-local and time-dependent behavior from transient thermal and mechanical driving forces. Comparing the physical significance of the Lagrange multipliers to the canonical ensemble suggests definitions of nonequilibrium ensembles at constant capacitance or inductance in addition to constant resistance. Our result is that statistical mechanical descriptions derived from a few primitive algebraic operations on information can be used to create experimentally-relevant and computable models. By construction, these models may use information from more detailed atomistic simulations. Two surprising consequences to be explored in further work are that (in)distinguishability factors are automatically predicted from the problem formulation and that a direct analogue of the second law for thermodynamic entropy production is found by considering information loss in stochastic processes. The information loss identifies a novel contribution from the instantaneous information entropy that ensures non-negative loss.
Figures
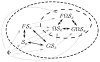
 , defining a coordinate space, and Ω, defining some further restriction are illustrated here. F and G are average value constraints, and their relative likelihoods can be calculated using (9) in either direction. For identical constraints, all hypotheses are completely connected, as shown by the double-headed, dark arrows. Restrictions such as
, defining a coordinate space, and Ω, defining some further restriction are illustrated here. F and G are average value constraints, and their relative likelihoods can be calculated using (9) in either direction. For identical constraints, all hypotheses are completely connected, as shown by the double-headed, dark arrows. Restrictions such as
 or Ω limit the set of propositions that can be directly compared without knowledge of P(Ω|I)/P(Φ|I), and only one comparison direction is allowed, illustrated by the grey, dotted arrows
or Ω limit the set of propositions that can be directly compared without knowledge of P(Ω|I)/P(Φ|I), and only one comparison direction is allowed, illustrated by the grey, dotted arrows
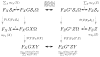
 → FX G
→ FX G
 Ω, there is a ‘reverse’ process FZ
Ω, there is a ‘reverse’ process FZ
 → FZ G*
→ FZ G*
 Ω signifying the dual maximum conditional entropy problem
Ω signifying the dual maximum conditional entropy problem
Similar articles
-
Nonequilibrium thermodynamics of interacting tunneling transport: variational grand potential, density functional formulation and nature of steady-state forces.J Phys Condens Matter. 2012 Oct 24;24(42):424219. doi: 10.1088/0953-8984/24/42/424219. Epub 2012 Oct 3. J Phys Condens Matter. 2012. PMID: 23032101
-
Steepest entropy ascent model for far-nonequilibrium thermodynamics: unified implementation of the maximum entropy production principle.Phys Rev E Stat Nonlin Soft Matter Phys. 2014 Oct;90(4):042113. doi: 10.1103/PhysRevE.90.042113. Epub 2014 Oct 7. Phys Rev E Stat Nonlin Soft Matter Phys. 2014. PMID: 25375444
-
Thermodynamic Langevin equations.Phys Rev E. 2024 Nov;110(5-1):054136. doi: 10.1103/PhysRevE.110.054136. Phys Rev E. 2024. PMID: 39690693
-
Entropy production in mesoscopic stochastic thermodynamics: nonequilibrium kinetic cycles driven by chemical potentials, temperatures, and mechanical forces.J Phys Condens Matter. 2016 Apr 20;28(15):153004. doi: 10.1088/0953-8984/28/15/153004. Epub 2016 Mar 17. J Phys Condens Matter. 2016. PMID: 26986039 Review.
-
Thermodynamics of stoichiometric biochemical networks in living systems far from equilibrium.Biophys Chem. 2005 Apr 22;114(2-3):213-20. doi: 10.1016/j.bpc.2004.12.001. Epub 2004 Dec 22. Biophys Chem. 2005. PMID: 15829355 Review.
Cited by
-
Deep neural network based quantum simulations and quasichemical theory for accurate modeling of molten salt thermodynamics.Chem Sci. 2022 Jun 15;13(28):8265-8273. doi: 10.1039/d2sc02227c. eCollection 2022 Jul 20. Chem Sci. 2022. PMID: 35919729 Free PMC article.
References
-
- Hille B. Ion Channels of Excitable Membranes. 3. Sinauer; Sunderland: 2001.
-
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch Eur J Physiol. 1981;391(2):85–100. - PubMed
-
- Jaynes ET. Predictive statistical mechanics. In: Moore GT, Scully MO, editors. Frontiers of Nonequilibrium Statistical Physics. Plenum Press; New York: 1986. p. 33.
-
- Gibbs JW. Elementary Principles in Statistical Mechanics. Scribner’s; New York: 1902.
Grants and funding
LinkOut - more resources
Full Text Sources
