Arterial Spin Labeling (ASL) fMRI: advantages, theoretical constrains, and experimental challenges in neurosciences
- PMID: 22966219
- PMCID: PMC3432878
- DOI: 10.1155/2012/818456
Arterial Spin Labeling (ASL) fMRI: advantages, theoretical constrains, and experimental challenges in neurosciences
Erratum in
- Int J Biomed Imaging. 2012;2012:658101
Abstract
Cerebral blood flow (CBF) is a well-established correlate of brain function and therefore an essential parameter for studying the brain at both normal and diseased states. Arterial spin labeling (ASL) is a noninvasive fMRI technique that uses arterial water as an endogenous tracer to measure CBF. ASL provides reliable absolute quantification of CBF with higher spatial and temporal resolution than other techniques. And yet, the routine application of ASL has been somewhat limited. In this review, we start by highlighting theoretical complexities and technical challenges of ASL fMRI for basic and clinical research. While underscoring the main advantages of ASL versus other techniques such as BOLD, we also expound on inherent challenges and confounds in ASL perfusion imaging. In closing, we expound on several exciting developments in the field that we believe will make ASL reach its full potential in neuroscience research.
Figures
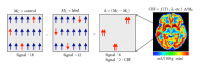
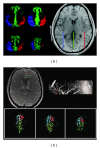

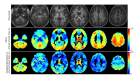
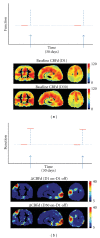
References
-
- Buxton R. Introduction to Functional Magnetic Resonance Imaging. Cambridge University Press; 2009.
-
- Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. Journal of Cerebral Blood Flow and Metabolism. 1996;16(6):1236–1249. - PubMed
-
- Kim DH, Spielman DM. Reducing gradient imperfections for spiral magnetic resonance spectroscopic imaging. Magnetic Resonance in Medicine. 2006;56(1):198–203. - PubMed
-
- Golay X, Hendrikse J, Lim TCC. Perfusion imaging using arterial spin labeling. Topics in Magnetic Resonance Imaging. 2004;15(1):10–27. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

