Molecular Regulation of the Mitochondrial F(1)F(o)-ATPsynthase: Physiological and Pathological Significance of the Inhibitory Factor 1 (IF(1))
- PMID: 22966230
- PMCID: PMC3433140
- DOI: 10.1155/2012/367934
Molecular Regulation of the Mitochondrial F(1)F(o)-ATPsynthase: Physiological and Pathological Significance of the Inhibitory Factor 1 (IF(1))
Abstract
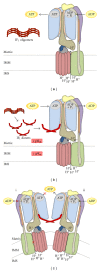
In mammals, the mitochondrial F(1)F(o)-ATPsynthase sets out the energy homeostasis by producing the bulk of cellular ATP. As for every enzyme, the laws of thermodynamics command it; however, it is privileged to have a dedicated molecular regulator that controls its rotation. This is the so-called ATPase Inhibitory Factor 1 (IF(1)) that blocks its reversal to avoid the consumption of cellular ATP when the enzyme acts as an ATP hydrolase. Recent evidence has also demonstrated that IF(1) may control the alignment of the enzyme along the mitochondrial inner membrane, thus increasing the interest for the molecule. We conceived this review to outline the fundamental knowledge of the F(1)F(o)-ATPsynthase and link it to the molecular mechanisms by which IF(1) regulates its way of function, with the ultimate goal to highlight this as an important and possibly unique means to control this indispensable enzyme in both physiological and pathological settings.
Figures


Similar articles
-
Neuroprotective coordination of cell mitophagy by the ATPase Inhibitory Factor 1.Pharmacol Res. 2016 Jan;103:56-68. doi: 10.1016/j.phrs.2015.10.010. Epub 2015 Oct 17. Pharmacol Res. 2016. PMID: 26484591
-
Structures and interactions of proteins involved in the coupling function of the protonmotive F(o)F(1)-ATP synthase.Curr Protein Pept Sci. 2002 Aug;3(4):451-60. doi: 10.2174/1389203023380558. Curr Protein Pept Sci. 2002. PMID: 12370007 Review.
-
Mitochondrial and cell-surface F0F1ATPsynthase in innate and acquired cardioprotection.J Bioenerg Biomembr. 2009 Apr;41(2):151-7. doi: 10.1007/s10863-009-9208-8. J Bioenerg Biomembr. 2009. PMID: 19387805 Review.
-
IF(1) distribution in HepG2 cells in relation to ecto-F(0)F (1)ATPsynthase and calmodulin.J Bioenerg Biomembr. 2007 Aug;39(4):291-300. doi: 10.1007/s10863-007-9091-0. Epub 2007 Sep 13. J Bioenerg Biomembr. 2007. PMID: 17851741
-
Mitochondria as ATP consumers in cellular pathology.Biochim Biophys Acta. 2010 Jan;1802(1):221-7. doi: 10.1016/j.bbadis.2009.08.008. Epub 2009 Aug 26. Biochim Biophys Acta. 2010. PMID: 19715757 Review.
Cited by
-
Protective mechanism of shenmai on myocardial ischemia-reperfusion through the energy metabolism pathway.Am J Transl Res. 2019 Jul 15;11(7):4046-4062. eCollection 2019. Am J Transl Res. 2019. PMID: 31396317 Free PMC article.
-
The miR-29 transcriptome in endocrine-sensitive and resistant breast cancer cells.Sci Rep. 2017 Jul 12;7(1):5205. doi: 10.1038/s41598-017-05727-w. Sci Rep. 2017. PMID: 28701793 Free PMC article.
-
Nutrition and Training Influences on the Regulation of Mitochondrial Adenosine Diphosphate Sensitivity and Bioenergetics.Sports Med. 2017 Mar;47(Suppl 1):13-21. doi: 10.1007/s40279-017-0693-3. Sports Med. 2017. PMID: 28332118 Free PMC article. Review.
-
The mitochondrial H(+)-ATP synthase and the lipogenic switch: new core components of metabolic reprogramming in induced pluripotent stem (iPS) cells.Cell Cycle. 2013 Jan 15;12(2):207-18. doi: 10.4161/cc.23352. Epub 2012 Jan 15. Cell Cycle. 2013. PMID: 23287468 Free PMC article. Review.
-
Inhibition of the mitochondrial ATPase function by IF1 changes the spatiotemporal organization of ATP synthase.Biochim Biophys Acta Bioenerg. 2021 Jan 1;1862(1):148322. doi: 10.1016/j.bbabio.2020.148322. Epub 2020 Oct 14. Biochim Biophys Acta Bioenerg. 2021. PMID: 33065099 Free PMC article.
References
-
- Appleby RD, Porteous WK, Hughes G, et al. Quantitation and origin of the mitochondrial membrane potential in human cells lacking mitochondrial DNA. European Journal of Biochemistry. 1999;262(1):108–116. - PubMed
-
- Martin J, Mahlke K, Pfanner N. Role of an energized inner membrane in mitochondrial protein import: δψ drives the movement of presequences. The Journal of Biological Chemistry. 1991;266(27):18051–18057. - PubMed
-
- Scorrano L, Ashiya M, Buttle K, et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Developmental Cell. 2002;2(1):55–67. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

