Periostin as a biomarker of the amniotic membrane
- PMID: 22966238
- PMCID: PMC3395182
- DOI: 10.1155/2012/987185
Periostin as a biomarker of the amniotic membrane
Erratum in
- Stem Cells Int. 2013;2013:509593. Camus, Anne [added]
Abstract
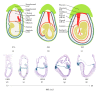
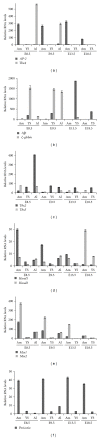
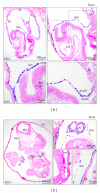
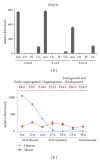
Tracing the precise developmental origin of amnion and amnion-derived stem cells is still challenging and depends chiefly on analyzing powerful genetic model amniotes like mouse. Profound understanding of the fundamental differences in amnion development in both the disc-shaped primate and human embryo and the cup-shaped mouse embryo is pivotal in particular when sampling amniotic membrane from nonprimate species for isolating candidate amniotic stem cells. The availability of molecular marker genes that are specifically expressed in the amniotic membrane and not in other extraembryonic membranes would be instrumental to validate unequivocally the starting material under investigation. So far such amniotic markers have not been reported. We postulated that bone morphogenetic protein (BMP) target genes are putative amniotic membrane markers mainly because deficiency in one of several components of the BMP signaling cascade in mice has been documented to result in defective development of the early amnion. Comparative gene expression analysis of acknowledged target genes for BMP in different extraembryonic tissues, combined with in situ hybridization, identified Periostin (Postn) mRNA enrichment in amnion throughout gestation. In addition, we identify and propose a combination of markers as transcriptional signature for the different extraembryonic tissues in mouse.
Figures
Similar articles
-
The development of the amnion in mice and other amniotes.Philos Trans R Soc Lond B Biol Sci. 2022 Dec 5;377(1865):20210258. doi: 10.1098/rstb.2021.0258. Epub 2022 Oct 17. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36252226 Free PMC article. Review.
-
Amniotic ectoderm expansion in mouse occurs via distinct modes and requires SMAD5-mediated signalling.Development. 2018 Jul 2;145(13):dev157222. doi: 10.1242/dev.157222. Development. 2018. PMID: 29884675 Free PMC article.
-
On the origin of amniotic stem cells: of mice and men.Int J Dev Biol. 2010;54(5):761-77. doi: 10.1387/ijdb.092935md. Int J Dev Biol. 2010. PMID: 20446274 Review.
-
The extended analogy of extraembryonic development in insects and amniotes.Philos Trans R Soc Lond B Biol Sci. 2022 Dec 5;377(1865):20210268. doi: 10.1098/rstb.2021.0268. Epub 2022 Oct 17. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36252225 Free PMC article. Review.
-
BMP-dependent serosa and amnion specification in the scuttle fly Megaselia abdita.Development. 2012 Sep;139(18):3373-82. doi: 10.1242/dev.083873. Epub 2012 Aug 8. Development. 2012. PMID: 22874914
Cited by
-
Recent advances in understanding cell types during human gastrulation.Semin Cell Dev Biol. 2022 Nov;131:35-43. doi: 10.1016/j.semcdb.2022.05.004. Epub 2022 May 21. Semin Cell Dev Biol. 2022. PMID: 35606274 Free PMC article. Review.
-
The development of the amnion in mice and other amniotes.Philos Trans R Soc Lond B Biol Sci. 2022 Dec 5;377(1865):20210258. doi: 10.1098/rstb.2021.0258. Epub 2022 Oct 17. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36252226 Free PMC article. Review.
-
Amniotic ectoderm expansion in mouse occurs via distinct modes and requires SMAD5-mediated signalling.Development. 2018 Jul 2;145(13):dev157222. doi: 10.1242/dev.157222. Development. 2018. PMID: 29884675 Free PMC article.
-
A pluripotent stem cell-based model for post-implantation human amniotic sac development.Nat Commun. 2017 Aug 8;8(1):208. doi: 10.1038/s41467-017-00236-w. Nat Commun. 2017. PMID: 28785084 Free PMC article.
-
Embryo model completes gastrulation to neurulation and organogenesis.Nature. 2022 Oct;610(7930):143-153. doi: 10.1038/s41586-022-05246-3. Epub 2022 Aug 25. Nature. 2022. PMID: 36007540 Free PMC article.
References
-
- Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the First International Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26(2):300–311. - PubMed
-
- Dobreva MP, Pereira PNG, Deprest J, Zwijsen A. On the origin of amniotic stem cells: of mice and men. International Journal of Developmental Biology. 2010;54(5):761–777. - PubMed
-
- Marcus AJ, Coyne TM, Rauch J, Woodbury D, Black IB. Isolation, characterization, and differentiation of stem cells derived from the rat amniotic membrane. Differentiation. 2008;76(2):130–144. - PubMed
-
- Ditadi A, De Coppi P, Picone O, et al. Human and murine amniotic fluid c-Kit+Lin- cells display hematopoietic activity. Blood. 2009;113(17):3953–3960. - PubMed
-
- Monteiro RM, Chuva de Sousa Lopes SM, Korchynskyi O, ten Dijke P, Mummery CL. Spatio-temporal activation of Smad1 and Smad5 in vivo: monitoring transcriptional activity of Smad proteins. Journal of Cell Science. 2004;117(20):4653–4663. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

