Retrospective analysis of Japanese patients with relapse or refractory small-cell lung cancer treated with amrubicin hydrochloride
- PMID: 22966345
- PMCID: PMC3436418
- DOI: 10.3892/ol_00000101
Retrospective analysis of Japanese patients with relapse or refractory small-cell lung cancer treated with amrubicin hydrochloride
Abstract
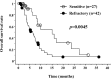
Amrubicin (AMR) is one of the most active chemotherapeutic agents for small-cell lung cancer (SCLC). Previous phase II studies reported on its effectiveness and severe hematological toxicities. However, AMR has yet to be approved outside Japan. Subsequently, no extensive evidence of its effects exist. Between January 2004 and October 2009, 69 patients received AMR for relapsed SCLC at our hospital. We reviewed these patients, and analyzed the efficacy and hematological toxicities of AMR. There were 27 sensitive relapses (S) and 42 refractory relapses (R). Patients received platinum agents, and 43 and 71% of the patients received etoposide and irinotecan, respectively. The median number of treatment cycles was 3 (range 1-14), and the response rate was 51% (70% in the S and 38% in the R cases, respectively). In patients administered with AMR as second-line therapy, the response rate was 55% and as third-line therapy, 39%. Median progression-free survival time was 3.2 months in the S and 1.9 months in the R patients (p=0.1071). Median survival time from the start of AMR was 6.2 months in the S and 4.8 months in the R cases (p=0.0045). The frequency of grade ≥3 hematological toxicities was leukopenia (41%), neutropenia (51%), anemia (14%), thrombocytopenia (17%) and febrile neutropenia (12%). No treatment-related death was observed. Although hematological toxicities, particularly neutropenia, were severe, AMR showed excellent anti-tumor activity, not only in the S, but also in the R cases, as shown in previous phase II studies. These results warrant further evaluation of AMR in the second-line setting, and also in the first-line setting in both limited- and extensive-stage disease. We conducted a phase II study to assess the efficacy of consolidation chemotherapy with AMR after standard chemoradiation in limited-stage SCLC.
Figures

Similar articles
-
A retrospective study of amrubicin monotherapy for the treatment of relapsed small cell lung cancer in elderly patients.Cancer Chemother Pharmacol. 2017 Sep;80(3):615-622. doi: 10.1007/s00280-017-3403-9. Epub 2017 Jul 31. Cancer Chemother Pharmacol. 2017. PMID: 28761968 Free PMC article.
-
Clinical outcome of amrubicin therapy according to the prior chemotherapy sensitivities of extensive small cell lung cancer.Osaka City Med J. 2011 Dec;57(2):59-66. Osaka City Med J. 2011. PMID: 22443079
-
Efficacy of Platinum-Based Chemotherapy for Relapsed Small-Cell Lung Cancer after Amrubicin Monotherapy in Elderly Patients and Patients with Poor Performance Status.Oncology. 2018;94(4):207-214. doi: 10.1159/000486038. Epub 2018 Jan 31. Oncology. 2018. PMID: 29393275
-
Relapsed small cell lung cancer: treatment options and latest developments.Ther Adv Med Oncol. 2014 Mar;6(2):69-82. doi: 10.1177/1758834013517413. Ther Adv Med Oncol. 2014. PMID: 24587832 Free PMC article. Review.
-
Efficacy and Safety of Amrubicin in Non-Small-Cell Lung Cancer Patients Beyond Third-Line Therapy.Oncol Res Treat. 2019;42(1-2):52-56. doi: 10.1159/000493199. Epub 2018 Dec 12. Oncol Res Treat. 2019. PMID: 30537755
Cited by
-
Efficacy and safety of amrubicin monotherapy after atezolizumab plus carboplatin and etoposide in patients with relapsed small-cell lung cancer.Invest New Drugs. 2022 Oct;40(5):1066-1079. doi: 10.1007/s10637-022-01269-9. Epub 2022 Jun 24. Invest New Drugs. 2022. PMID: 35749041 Free PMC article.
-
Efficacy of different monotherapies in second-line treatment for small cell lung cancer: a meta-analysis of randomized controlled trials.Int J Clin Exp Med. 2015 Oct 15;8(10):19689-700. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26770633 Free PMC article.
-
A retrospective study of amrubicin monotherapy for the treatment of relapsed small cell lung cancer in elderly patients.Cancer Chemother Pharmacol. 2017 Sep;80(3):615-622. doi: 10.1007/s00280-017-3403-9. Epub 2017 Jul 31. Cancer Chemother Pharmacol. 2017. PMID: 28761968 Free PMC article.
References
-
- Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366:1385–1396. - PubMed
-
- O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441–5447. - PubMed
-
- Von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–667. - PubMed
-
- Inoue K, Ogawa M, Horikoshi N, et al. Phase I and pharmacokinetic study of SM-5887, a new anthracycline derivative. Invest New Drugs. 1989;7:213–218. - PubMed
-
- Negoro S, Fukuoka M, Nakamura S, et al. Phase I-II study of amrubicin (SM-5887), a novel 9-aminoanthracycline, by iv administration for 3 consecutive days in patients with advanced non-small cell lung cancer. Proc Am Soc Clin Oncol. 1995;14:361.
LinkOut - more resources
Full Text Sources
