Riluzole-triggered GSH synthesis via activation of glutamate transporters to antagonize methylmercury-induced oxidative stress in rat cerebral cortex
- PMID: 22966415
- PMCID: PMC3432391
- DOI: 10.1155/2012/534705
Riluzole-triggered GSH synthesis via activation of glutamate transporters to antagonize methylmercury-induced oxidative stress in rat cerebral cortex
Abstract
Objective: This study was to evaluate the effect of riluzole on methylmercury- (MeHg-) induced oxidative stress, through promotion of glutathione (GSH) synthesis by activating of glutamate transporters (GluTs) in rat cerebral cortex.
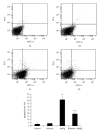
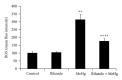
Methods: Eighty rats were randomly assigned to four groups, control group, riluzole alone group, MeHg alone group, and riluzole + MeHg group. The neurotoxicity of MeHg was observed by measuring mercury (Hg) absorption, pathological changes, and cell apoptosis of cortex. Oxidative stress was evaluated via determining reactive oxygen species (ROS), 8-hydroxy-2-deoxyguanosine (8-OHdG), malondialdehyde (MDAs), carbonyl, sulfydryl, and GSH in cortex. Glutamate (Glu) transport was studied by measuring Glu, glutamine (Gln), mRNA, and protein of glutamate/aspartate transporter (GLAST) and glutamate transporter-1 (GLT-1).
Result: (1) MeHg induced Hg accumulation, pathological injury, and apoptosis of cortex; (2) MeHg increased ROS, 8-OHdG, MDA, and carbonyl, and inhibited sulfydryl and GSH; (3) MeHg elevated Glu, decreased Gln, and downregulated GLAST and GLT-1 mRNA expression and protein levels; (4) riluzole antagonized MeHg-induced downregulation of GLAST and GLT-1 function and expression, GSH depletion, oxidative stress, pathological injury, and apoptosis obviously.
Conclusion: Data indicate that MeHg administration induced oxidative stress in cortex and that riluzole could antagonize this situation through elevation of GSH synthesis by activating of GluTs.
Figures




Similar articles
-
Exploring cross-talk between oxidative damage and excitotoxicity and the effects of riluzole in the rat cortex after exposure to methylmercury.Neurotox Res. 2014 Jul;26(1):40-51. doi: 10.1007/s12640-013-9448-6. Epub 2014 Feb 12. Neurotox Res. 2014. PMID: 24519665
-
Excitotoxicity and oxidative damages induced by methylmercury in rat cerebral cortex and the protective effects of tea polyphenols.Environ Toxicol. 2014 Mar;29(3):269-83. doi: 10.1002/tox.21755. Epub 2012 Jan 5. Environ Toxicol. 2014. PMID: 22223486
-
Sulforaphane Prevents Methylmercury-Induced Oxidative Damage and Excitotoxicity Through Activation of the Nrf2-ARE Pathway.Mol Neurobiol. 2017 Jan;54(1):375-391. doi: 10.1007/s12035-015-9643-y. Epub 2016 Jan 7. Mol Neurobiol. 2017. PMID: 26742517
-
Glutamate Transporters/Na(+), K(+)-ATPase Involving in the Neuroprotective Effect as a Potential Regulatory Target of Glutamate Uptake.Mol Neurobiol. 2016 Mar;53(2):1124-1131. doi: 10.1007/s12035-014-9071-4. Epub 2015 Jan 14. Mol Neurobiol. 2016. PMID: 25586061 Review.
-
Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity.Braz J Med Biol Res. 2007 Mar;40(3):285-91. doi: 10.1590/s0100-879x2007000300001. Braz J Med Biol Res. 2007. PMID: 17334523 Review.
Cited by
-
The Effects of Riluzole on Cisplatin-induced Ototoxicity.Int Arch Otorhinolaryngol. 2019 Jul;23(3):e267-e275. doi: 10.1055/s-0038-1676654. Epub 2019 Mar 1. Int Arch Otorhinolaryngol. 2019. PMID: 31360245 Free PMC article.
-
Differential Effects of Human P301L Tau Expression in Young versus Aged Mice.Int J Mol Sci. 2021 Oct 28;22(21):11637. doi: 10.3390/ijms222111637. Int J Mol Sci. 2021. PMID: 34769068 Free PMC article.
-
Melatonin Blocks Morphine-Induced Place Preference: Involvement of GLT-1, NF-κB, BDNF, and CREB in the Nucleus Accumbens.Front Behav Neurosci. 2021 Oct 14;15:762297. doi: 10.3389/fnbeh.2021.762297. eCollection 2021. Front Behav Neurosci. 2021. PMID: 34720901 Free PMC article.
-
The Potential for Natural Antioxidant Supplementation in the Early Stages of Neurodegenerative Disorders.Int J Mol Sci. 2020 Apr 9;21(7):2618. doi: 10.3390/ijms21072618. Int J Mol Sci. 2020. PMID: 32283806 Free PMC article. Review.
-
Leptin level and oxidative stress contribute to obesity-induced low testosterone in murine testicular tissue.Oxid Med Cell Longev. 2014;2014:190945. doi: 10.1155/2014/190945. Epub 2014 Apr 14. Oxid Med Cell Longev. 2014. PMID: 24829619 Free PMC article.
References
-
- Turner MD, Marsh DO, Smith JC, et al. Methylmercury in populations eating large quantities of marine fish. Archives of Environmental Health. 1980;35(6):367–378. - PubMed
-
- Aschner M, Syversen T, Souza DO, Rocha JBT, Farina M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Brazilian Journal of Medical and Biological Research. 2007;40(3):285–291. - PubMed
-
- Shanker G, Aschner M. Methylmercury-induced reactive oxygen species formation in neonatal cerebral astrocytic cultures is attenuated by antioxidants. Molecular Brain Research. 2003;110(1):85–91. - PubMed
-
- Yee S, Choi BH. Oxidative stress in neurotoxic effects of methylmercury poisoning. NeuroToxicology. 1996;17(1):17–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

