Loss of asthma control in pediatric patients after discontinuation of long-acting Beta-agonists
- PMID: 22966431
- PMCID: PMC3432548
- DOI: 10.1155/2012/894063
Loss of asthma control in pediatric patients after discontinuation of long-acting Beta-agonists
Abstract
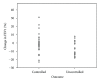
Recent asthma recommendations advocate the use of long-acting beta-agonists (LABAs) in uncontrolled asthma, but also stress the importance of stepping down this therapy once asthma control has been achieved. The objective of this study was to evaluate downtitration of LABA therapy in pediatric patients who are well-controlled on combination-inhaled corticosteroid (ICS)/LABA therapy. Clinical and physiologic outcomes were studied in children with moderate-to-severe persistent asthma after switching from combination (ICS/LABA) to monotherapy with ICS. Of the 54 patients, 34 (63%) were determined to have stable asthma after the switch, with a mean followup of 10.7 weeks. Twenty (37%) had loss of asthma control leading to addition of leukotriene receptor antagonists, increased ICS, or restarting LABA. There were 2 exacerbations requiring treatment with systemic steroids. In patients with loss of control, there was a statistically significant decline in FEV(1) (-8% versus -1.9%, P = 0.03) and asthma control test (-3.2 versus -0.5, P = 0.03). This did not approach significance for FEF(25-75%), exhaled nitric oxide, lung volumes or airway reactivity. No demographic, asthma control measures, or lung function variables predicted loss of control. Pediatric patients with moderate-to-severe persistent asthma who discontinue LABA therapy have a 37% chance of losing asthma control resulting in augmented maintenance therapies. Recent recommendations of discontinuing LABA therapy as soon as control is achieved should be evaluated in a prospective long-term study.
Figures


References
-
- Cochrane Review 2010. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. http://www.thecochranelibrary.com/userfiles/ccoch/file/CD005535.pdf. - PMC - PubMed
-
- Bisgaard H, Szefler S. Long-acting β2 agonists and paediatric asthma. The Lancet. 2006;367(9507):286–288. - PubMed
-
- Friedman HS, Eid NS, Crespi S, Wilcox TK, Reardon G. Retrospective claims study of fluticasone propionate/salmeterol fixed-dose combination use as initial asthma controller therapy in children despite guideline recommendations. Clinical Therapeutics. 2009;31(5):1056–1063. - PubMed
-
- Chowdhury BA, Pan GD. The FDA and safe use of long-acting beta-agonists in the treatment of asthma. The New England Journal of Medicine. 2010;362(13):1169–1171. - PubMed
-
- Nelson HS, Weiss ST, Bleecker EK, Yancey SW, Dorinsky PM. The salmeterol multicenter asthma research trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129(1):15–26. - PubMed
LinkOut - more resources
Full Text Sources

