Combination therapy of interferon Beta-1b and tacrolimus: a pilot safety study
- PMID: 22966460
- PMCID: PMC3431088
- DOI: 10.1155/2012/935921
Combination therapy of interferon Beta-1b and tacrolimus: a pilot safety study
Abstract
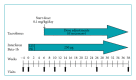
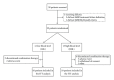
Tacrolimus is a calcineurin inhibitor which works to induce immune suppression by preventing cytokine transcription and lymphocyte activation. Combining the immunomodulator interferon beta-1b (Betaseron) with the immunosuppressant tacrolimus (Prograf) may have the potential of additive therapeutic benefit through the complementary mechanisms of action of these two therapeutics. In this randomized, open-label, multicenter, two-arm pilot study, the authors examined the safety and tolerability of the combination of interferon beta-1b and tacrolimus in relapsing remitting (RRMS) and secondary progressive (SPMS) multiple sclerosis patients who have failed one or more immunomodulatory therapies. Patients (n = 25) received a combination of interferon beta-1b subcutaneously every other day and oral tacrolimus (low blood level tacrolimus, 1-5 ng/mL, or high blood level tacrolimus, 5-10 ng/mL) for a period of 38 weeks. The combination therapy of interferon beta-1b and tacrolimus over the 10-month period of the study was shown to be safe and relatively well tolerated. There were no unexpected adverse events occurring as the result of the combination therapy. Further study of this combination therapy in patients with multiple sclerosis unresponsive to conventional therapy is warranted.
Figures
Similar articles
-
Spotlight on Interferon-beta-1b in relapsing-remitting and secondary progressive multiple sclerosis.BioDrugs. 2004;18(5):343-7. doi: 10.2165/00063030-200418050-00006. BioDrugs. 2004. PMID: 15377176 Review.
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
-
Interferon-beta-1b: a review of its use in relapsing-remitting and secondary progressive multiple sclerosis.CNS Drugs. 2004;18(8):521-46. doi: 10.2165/00023210-200418080-00004. CNS Drugs. 2004. PMID: 15182221 Review.
-
Interferon-β-1b: a review of its use in multiple sclerosis.CNS Drugs. 2011 Jan;25(1):67-88. doi: 10.2165/11206430-000000000-00000. CNS Drugs. 2011. PMID: 21128695 Review.
-
Tolerability and safety profile of 12- to 28-week treatment with interferon beta-1b 250 and 500 microg QOD in patients with relapsing-remitting multiple sclerosis: a multicenter, randomized, double-blind, parallel-group pilot study.Clin Ther. 2008 Jun;30(6):1102-12. doi: 10.1016/j.clinthera.2008.06.013. Clin Ther. 2008. PMID: 18640466 Clinical Trial.
Cited by
-
Treatment approaches to patients with multiple sclerosis and coexisting autoimmune disorders.Ther Adv Neurol Disord. 2021 Aug 23;14:17562864211035542. doi: 10.1177/17562864211035542. eCollection 2021. Ther Adv Neurol Disord. 2021. PMID: 34457039 Free PMC article. Review.
-
The Anti-Inflammatory Effects of Oral-Formulated Tacrolimus in Mice with Experimental Autoimmune Encephalomyelitis.J Korean Med Sci. 2017 Sep;32(9):1502-1507. doi: 10.3346/jkms.2017.32.9.1502. J Korean Med Sci. 2017. PMID: 28776347 Free PMC article.
-
FK506 and Lactobacillus acidophilus ameliorate acute graft-versus-host disease by modulating the T helper 17/regulatory T-cell balance.J Transl Med. 2022 Feb 25;20(1):104. doi: 10.1186/s12967-022-03303-z. J Transl Med. 2022. PMID: 35216600 Free PMC article.
-
Genetic susceptibility and causal pathway analysis of eye disorders coexisting in multiple sclerosis.Front Immunol. 2024 Feb 5;15:1337528. doi: 10.3389/fimmu.2024.1337528. eCollection 2024. Front Immunol. 2024. PMID: 38375484 Free PMC article.
References
-
- Bar-Or A. The immunology of multiple sclerosis. Seminars in Neurology. 2008;28(1):29–45. - PubMed
-
- Boggild M. Rationale and experience with combination therapies in multiple sclerosis. Journal of Neurology. 2006;253(6):VI/45–VI/51.
-
- Knobler RL, Greenstein JI, Johnson KP, et al. Systemic recombinant human interferon-β treatment of relapsing-remitting multiple sclerosis: pilot study analysis and six-year follow-up. Journal of Interferon Research. 1993;13(5):333–340. - PubMed
-
- The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43(4):655–661. - PubMed
-
- Paty DW, Li DKB. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo- controlled trial. Neurology. 1993;43(4):662–667. - PubMed
LinkOut - more resources
Full Text Sources

