Density variant glycan microarray for evaluating cross-linking of mucin-like glycoconjugates by lectins
- PMID: 22967056
- PMCID: PMC3458438
- DOI: 10.1021/ja302193u
Density variant glycan microarray for evaluating cross-linking of mucin-like glycoconjugates by lectins
Abstract
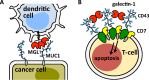
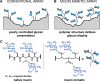
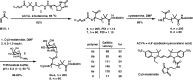
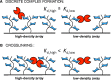
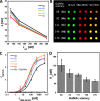
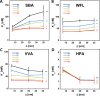
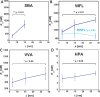
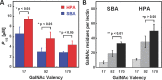
Interactions of mucin glycoproteins with cognate receptors are dictated by the structures and spatial organization of glycans that decorate the mucin polypeptide backbone. The glycan-binding proteins, or lectins, that interact with mucins are often oligomeric receptors with multiple ligand binding domains. In this work, we employed a microarray platform comprising synthetic glycopolymers that emulate natural mucins arrayed at different surface densities to evaluate how glycan valency and spatial separation affect the preferential binding mode of a particular lectin. We evaluated a panel of four lectins (Soybean agglutinin (SBA), Wisteria floribunda lectin (WFL), Vicia villosa-B-4 agglutinin (VVA), and Helix pomatia agglutin (HPA)) with specificity for α-N-acetylgalactosamine (α-GalNAc), an epitope displayed on mucins overexpressed in many adenocarcinomas. While these lectins possess the ability to agglutinate A(1)-blood cells carrying the α-GalNAc epitope and cross-link low valency glycoconjugates, only SBA showed a tendency to form intermolecular cross-links among the arrayed polyvalent mucin mimetics. These results suggest that glycopolymer microarrays can reveal discrete higher-order binding preferences beyond the recognition of individual glycan epitopes. Our findings indicate that glycan valency can set thresholds for cross-linking by lectins. More broadly, well-defined synthetic glycopolymers enable the integration of glycoconjugate structural and spatial diversity in a single microarray screening platform.
Figures

Similar articles
-
Mechanism of multivalent glycoconjugate-lectin interaction: An update.Adv Carbohydr Chem Biochem. 2023;84:1-21. doi: 10.1016/bs.accb.2023.10.004. Epub 2023 Nov 10. Adv Carbohydr Chem Biochem. 2023. PMID: 37979977
-
Comparison of the carbohydrate-binding specificities of seven N-acetyl-D-galactosamine-recognizing lectins.Eur J Biochem. 1990 Jul 31;191(2):461-6. doi: 10.1111/j.1432-1033.1990.tb19144.x. Eur J Biochem. 1990. PMID: 2384093
-
Analysis of glycoconjugate patterns of normal and hormone-induced dysplastic Noble rat prostates, and an androgen-independent Noble rat prostate tumor, by lectin histochemistry and protein blotting.Prostate. 2001 Jan 1;46(1):21-32. doi: 10.1002/1097-0045(200101)46:1<21::aid-pros1004>3.0.co;2-g. Prostate. 2001. PMID: 11170128
-
Differential binding properties of Gal/GalNAc specific lectins available for characterization of glycoreceptors.Indian J Biochem Biophys. 1997 Feb-Apr;34(1-2):61-71. Indian J Biochem Biophys. 1997. PMID: 9343930 Review.
-
Detection of inflammation- and neoplasia-associated alterations in human large intestine using plant/invertebrate lectins, galectin-1 and neoglycoproteins.Acta Anat (Basel). 1998;161(1-4):219-33. doi: 10.1159/000046460. Acta Anat (Basel). 1998. PMID: 9780361 Review.
Cited by
-
Synthesis and biomedical applications of mucin mimic materials.Adv Drug Deliv Rev. 2022 Dec;191:114540. doi: 10.1016/j.addr.2022.114540. Epub 2022 Oct 10. Adv Drug Deliv Rev. 2022. PMID: 36228896 Free PMC article. Review.
-
Identification of Nanomolar Lectin Ligands by a Glycodendrimer Microarray.ACS Omega. 2018 Oct 31;3(10):14013-14020. doi: 10.1021/acsomega.8b01526. Epub 2018 Oct 25. ACS Omega. 2018. PMID: 30411056 Free PMC article.
-
On-Chip Neo-Glycopeptide Synthesis for Multivalent Glycan Presentation.Chemistry. 2020 Aug 6;26(44):9954-9963. doi: 10.1002/chem.202001291. Epub 2020 Jun 11. Chemistry. 2020. PMID: 32315099 Free PMC article.
-
The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans.Nat Rev Drug Discov. 2021 Mar;20(3):217-243. doi: 10.1038/s41573-020-00093-1. Epub 2021 Jan 18. Nat Rev Drug Discov. 2021. PMID: 33462432 Free PMC article. Review.
-
Toward Glycomaterials with Selectivity as Well as Affinity.JACS Au. 2021 Oct 11;1(12):2089-2099. doi: 10.1021/jacsau.1c00352. eCollection 2021 Dec 27. JACS Au. 2021. PMID: 34984416 Free PMC article. Review.
References
-
- Lundquist J. J.; Toone E. J. Chem. Rev. 2002, 102, 555–578. - PubMed
- Mammen M.; Choi S.-K.; Whitesides G. M. Angew. Chem., Int. Ed. 1998, 37, 2754–2794. - PubMed
- Lee R. T.; Lee Y. C. Glycoconjugate J. 2000, 17, 543–551. - PubMed
- Kitov P. I.; Bundle D. R. J. Am. Chem. Soc. 2003, 125, 16271–16284. - PubMed
-
- Dam T. K.; Brewer C. F. Glycobiology 2010, 20, 270–279. - PubMed
-
- Weis W. I.; Taylor M. E.; Drickamer K. Immunol. Rev. 1998, 163, 19–34. - PubMed
-
- Saeland E.; van Vliet S. J.; Bäckström M.; van den Berg V. C. M.; Geijtenbeek T. B. H.; Meijer G. A.; van Kooyk Y. Cancer Immunol. Immunother. 2007, 56, 1225–1236. - PMC - PubMed
- Napoletano C.; Rughetti A.; Agervig Tarp M. P. A.; Coleman J.; Bennet P. E.; Picco G.; Sale P.; Denda-Nagai K.; Irimura T.; Mandel U.; Clausen H.; Frati L.; Taylor-Papadimitriou J.; Burchell J.; Nuti M. Cancer Res. 2007, 67, 8358–8367. - PubMed
-
- Hollingsworth M. A.; Swanson B. J. Nat. Rev. Cancer 2004, 4, 45–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

