Density variant glycan microarray for evaluating cross-linking of mucin-like glycoconjugates by lectins
- PMID: 22967056
- PMCID: PMC3458438
- DOI: 10.1021/ja302193u
Density variant glycan microarray for evaluating cross-linking of mucin-like glycoconjugates by lectins
Abstract
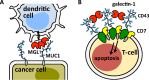
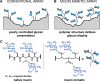
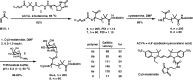
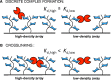
Interactions of mucin glycoproteins with cognate receptors are dictated by the structures and spatial organization of glycans that decorate the mucin polypeptide backbone. The glycan-binding proteins, or lectins, that interact with mucins are often oligomeric receptors with multiple ligand binding domains. In this work, we employed a microarray platform comprising synthetic glycopolymers that emulate natural mucins arrayed at different surface densities to evaluate how glycan valency and spatial separation affect the preferential binding mode of a particular lectin. We evaluated a panel of four lectins (Soybean agglutinin (SBA), Wisteria floribunda lectin (WFL), Vicia villosa-B-4 agglutinin (VVA), and Helix pomatia agglutin (HPA)) with specificity for α-N-acetylgalactosamine (α-GalNAc), an epitope displayed on mucins overexpressed in many adenocarcinomas. While these lectins possess the ability to agglutinate A(1)-blood cells carrying the α-GalNAc epitope and cross-link low valency glycoconjugates, only SBA showed a tendency to form intermolecular cross-links among the arrayed polyvalent mucin mimetics. These results suggest that glycopolymer microarrays can reveal discrete higher-order binding preferences beyond the recognition of individual glycan epitopes. Our findings indicate that glycan valency can set thresholds for cross-linking by lectins. More broadly, well-defined synthetic glycopolymers enable the integration of glycoconjugate structural and spatial diversity in a single microarray screening platform.
Figures
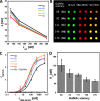
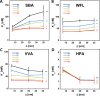
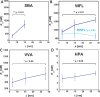
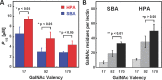

References
-
- Lundquist J. J.; Toone E. J. Chem. Rev. 2002, 102, 555–578. - PubMed
- Mammen M.; Choi S.-K.; Whitesides G. M. Angew. Chem., Int. Ed. 1998, 37, 2754–2794. - PubMed
- Lee R. T.; Lee Y. C. Glycoconjugate J. 2000, 17, 543–551. - PubMed
- Kitov P. I.; Bundle D. R. J. Am. Chem. Soc. 2003, 125, 16271–16284. - PubMed
-
- Dam T. K.; Brewer C. F. Glycobiology 2010, 20, 270–279. - PubMed
-
- Weis W. I.; Taylor M. E.; Drickamer K. Immunol. Rev. 1998, 163, 19–34. - PubMed
-
- Saeland E.; van Vliet S. J.; Bäckström M.; van den Berg V. C. M.; Geijtenbeek T. B. H.; Meijer G. A.; van Kooyk Y. Cancer Immunol. Immunother. 2007, 56, 1225–1236. - PMC - PubMed
- Napoletano C.; Rughetti A.; Agervig Tarp M. P. A.; Coleman J.; Bennet P. E.; Picco G.; Sale P.; Denda-Nagai K.; Irimura T.; Mandel U.; Clausen H.; Frati L.; Taylor-Papadimitriou J.; Burchell J.; Nuti M. Cancer Res. 2007, 67, 8358–8367. - PubMed
-
- Hollingsworth M. A.; Swanson B. J. Nat. Rev. Cancer 2004, 4, 45–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

