Albumin is taken up by hippocampal NG2 cells and astrocytes and decreases gap junction coupling
- PMID: 22967085
- PMCID: PMC3651829
- DOI: 10.1111/j.1528-1167.2012.03665.x
Albumin is taken up by hippocampal NG2 cells and astrocytes and decreases gap junction coupling
Abstract
Purpose: Dysfunction of the blood-brain barrier (BBB) and albumin extravasation have been suggested to play a role in the etiology of human epilepsy. In this context, dysfunction of glial cells attracts increasing attention. Our study was aimed to analyze in the hippocampus (1) which cell types internalize albumin injected into the lateral ventricle in vivo, (2) whether internalization into astrocytes impacts their coupling and expression of connexin 43 (Cx43), and (3) whether expression of Kir4.1, the predominating astrocytic K(+) channel subunit, is altered by albumin.
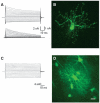
Methods: The patch-clamp method was combined with single cell tracer filling to investigate electrophysiologic properties and gap junction coupling (GJC). For cell identification, mice with cell type-specific expression of a fluorescent protein (NG2kiEYFP mice) and immunohistochemistry were employed. Semiquantitative real time polymerase chain reaction (RT-PCR) allowed analysis of Kir4.1 and Cx43 transcript levels.
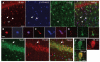
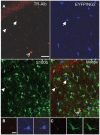
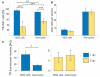
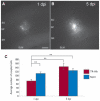
Key findings: We show that fluorescently labeled albumin is taken up by astrocytes, NG2 cells, and neurons, with NG2 cells standing out in terms of the quantity of uptake. Within 5 days postinjection (dpi), intracellular albumin accumulation was largely reduced suggesting rapid degradation. Electrophysiologic analysis of astrocytes and NG2 cells revealed no changes in their membrane properties at either time point. However, astrocytic GJC was significantly decreased at 1 dpi but returned to control levels within 5 dpi. We found no changes in hippocampal Cx43 transcript expression, suggesting that other mechanisms account for the observed changes in coupling. Kir4.1 mRNA was regulated oppositely in the CA1 stratum radiatum, with a strong increase at 1 dpi followed by a decrease at 5 dpi.
Significance: The present study demonstrates that extravasal albumin in the hippocampus induces rapid changes of astrocyte function, which can be expected to impair ion and transmitter homeostasis and contribute to hyperactivity and epileptogenesis. Therefore, astrocytes may represent alternative targets for antiepileptic therapeutic approaches.
Wiley Periodicals, Inc. © 2012 International League Against Epilepsy.
Figures
References
-
- Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49:318–338. - PubMed
-
- Bordey A, Sontheimer H. Postnatal development of ionic currents in rat hippocampal astrocytes in situ. J Neurophysiol. 1997;78:461–477. - PubMed
-
- Boucsein C, Kettenmann H, Nolte C. Electrophysiological properties of microglial cells in normal and pathologic rat brain slices. Eur J Neurosci. 2000;12:2049–2058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

