Phosphatidylinositol 3-kinase is an upstream regulator of the phosphodiesterase 3B pathway of leptin signalling that may not involve activation of Akt in the rat hypothalamus
- PMID: 22967108
- PMCID: PMC3549038
- DOI: 10.1111/j.1365-2826.2012.02386.x
Phosphatidylinositol 3-kinase is an upstream regulator of the phosphodiesterase 3B pathway of leptin signalling that may not involve activation of Akt in the rat hypothalamus
Abstract
Leptin, the product of the obese gene, regulates energy homeostasis by acting primarily at the level of the hypothalamus. Leptin action through its receptor involves various pathways, including the signal transducer and activator of transcription (STAT)3, phosphatidylinositol 3-kinase (PI3K), and phosphodiesterase 3B (PDE3B)-cAMP signalling in the central nervous system and peripheral tissues. In the hypothalamus, leptin stimulates STAT3 activation, and induces PI3K and PDE3B activities, among others. We have previously demonstrated that PDE3B activation in the hypothalamus is critical for transducing the anorectic and body weight reducing effects of leptin. Similarly, PI3K has been implicated to play a critical role in leptin signalling in the hypothalamus. Although, in the insulin signalling pathway, PI3K is known to be an upstream regulator of PDE3B in non-neuronal tissues, it is still unknown whether this is also the case for leptin signalling in the hypothalamus. To address this possibility, the effect of wortmannin, a specific PI3K inhibitor, was examined on leptin-induced PDE3B activity in the hypothalamus of male rats. Intracerebroventricular injection of leptin (4 μg) significantly increased PDE3B activity by two-fold in the hypothalamus as expected. However, previous administration of wortmannin completely reversed the stimulatory effect of leptin on PDE3B activity in the hypothalamus. To investigate whether leptin stimulates phospho (p)-Akt levels and that there might be a possible upstream regulator of PDE3B, we examined the effects of i.c.v. leptin on p-Akt levels in the hypothalamus and compared them with the known stimulatory effect of insulin on p-Akt. We observed that insulin increased p-Akt levels but leptin failed to do so, although it increased p-STAT3 levels, in the rat hypothalamus. Immunocytochemistry confirmed the biochemical findings in that leptin failed but insulin increased the number of p-Akt positive cells in various hypothalamic nuclei. Taken together, these results implicate PI3K but not Akt as an upstream regulator of the PDE3B pathway of leptin signalling in the rat hypothalamus.
© 2012 British Society for Neuroendocrinology.
Figures
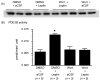
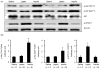
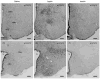





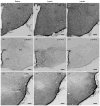
Similar articles
-
Phosphodiesterase-3B-cAMP pathway of leptin signalling in the hypothalamus is impaired during the development of diet-induced obesity in FVB/N mice.J Neuroendocrinol. 2015 Apr;27(4):293-302. doi: 10.1111/jne.12266. J Neuroendocrinol. 2015. PMID: 25702569
-
Hypothalamic Phosphodiesterase 3B Pathway Mediates Anorectic and Body Weight-Reducing Effects of Insulin in Male Mice.Neuroendocrinology. 2017;104(2):145-156. doi: 10.1159/000445523. Epub 2016 Mar 23. Neuroendocrinology. 2017. PMID: 27002827 Free PMC article.
-
Hypothalamic phosphatidylinositol 3-kinase-phosphodiesterase 3B-cyclic AMP pathway of leptin signalling is impaired following chronic central leptin infusion.J Neuroendocrinol. 2005 Nov;17(11):720-6. doi: 10.1111/j.1365-2826.2005.01362.x. J Neuroendocrinol. 2005. PMID: 16219000
-
Intracellular leptin-signaling pathways in hypothalamic neurons: the emerging role of phosphatidylinositol-3 kinase-phosphodiesterase-3B-cAMP pathway.Neuroendocrinology. 2011;93(4):201-10. doi: 10.1159/000326785. Epub 2011 Apr 5. Neuroendocrinology. 2011. PMID: 21464566 Free PMC article. Review.
-
Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance.Front Neuroendocrinol. 2003 Dec;24(4):225-53. doi: 10.1016/j.yfrne.2003.10.001. Front Neuroendocrinol. 2003. PMID: 14726256 Review.
Cited by
-
Chronic Timed Sleep Restriction Attenuates LepRb-Mediated Signaling Pathways and Circadian Clock Gene Expression in the Rat Hypothalamus.Front Neurosci. 2020 Sep 8;14:909. doi: 10.3389/fnins.2020.00909. eCollection 2020. Front Neurosci. 2020. PMID: 33013300 Free PMC article.
-
Evidence suggesting phosphodiesterase-3B regulation of NPY/AgRP gene expression in mHypoE-46 hypothalamic neurons.Neurosci Lett. 2015 Sep 14;604:113-8. doi: 10.1016/j.neulet.2015.08.003. Epub 2015 Aug 4. Neurosci Lett. 2015. PMID: 26254161 Free PMC article.
-
PI3 kinases p110α and PI3K-C2β negatively regulate cAMP via PDE3/8 to control insulin secretion in mouse and human islets.Mol Metab. 2016 May 11;5(7):459-471. doi: 10.1016/j.molmet.2016.05.003. eCollection 2016 Jul. Mol Metab. 2016. PMID: 27408772 Free PMC article.
-
Molecular Mechanisms of Hypothalamic Insulin Resistance.Int J Mol Sci. 2019 Mar 15;20(6):1317. doi: 10.3390/ijms20061317. Int J Mol Sci. 2019. PMID: 30875909 Free PMC article. Review.
-
Leptin signaling and leptin resistance.Med Rev (2021). 2022 Aug 9;2(4):363-384. doi: 10.1515/mr-2022-0017. eCollection 2022 Aug. Med Rev (2021). 2022. PMID: 37724323 Free PMC article. Review.
References
-
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. - PubMed
-
- Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol. 2003;24:225–53. - PubMed
-
- Sahu A. Minireview: A hypothalamic role in energy balance with special emphasis on leptin. Endocrinology. 2004;145:2613–20. - PubMed
-
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95. - PubMed
-
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

