Denosumab: mechanism of action and clinical outcomes
- PMID: 22967310
- PMCID: PMC3549483
- DOI: 10.1111/ijcp.12022
Denosumab: mechanism of action and clinical outcomes
Abstract
Aims: To describe the mechanisms of action of denosumab, a novel antiresorptive agent, contrasting it with other antiresorptive and anabolic osteoporosis treatments.
Methods: Published papers related to the mechanism of action of approved osteoporosis treatments were sought through MEDLINE searches.
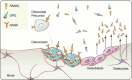
Findings: Osteoporotic fractures carry a substantial burden of morbidity and mortality, but pharmacotherapy can prevent such fractures in high-risk individuals. Antiresorptive drugs (e.g. bisphosphonates, oestrogen, denosumab) reduce bone turnover by distinct mechanisms. Denosumab, a recently approved therapy, is a fully human monoclonal antibody that binds the cytokine RANKL (receptor activator of NFκB ligand), an essential factor initiating bone turnover. RANKL inhibition blocks osteoclast maturation, function and survival, thus reducing bone resorption. In contrast, bisphosphonates bind bone mineral, where they are absorbed by mature osteoclasts, inducing osteoclast apoptosis and suppressing resorption. These differences in mechanism influence both the onset and reversibility of treatment.
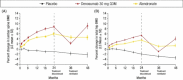
Discussion: Effective pharmacotherapy is necessary for patients at high risk of fracture. Among the treatment options for postmenopausal osteoporosis, there are significant differences in mechanism and dosing. Denosumab acts by a novel mechanism and is administered twice yearly by subcutaneous injection. Identified by Osteoporosis Canada Clinical Practice Guidelines as a first-line agent for treatment of postmenopausal osteoporosis, denosumab represents an important addition to our treatment options.
© 2012 Blackwell Publishing Ltd.
Figures

Comment in
-
Denosumab: do we need another of the same?Int J Clin Pract. 2012 Dec;66(12):1129-31. doi: 10.1111/ijcp.12036. Int J Clin Pract. 2012. PMID: 23163493 No abstract available.
References
-
- Rizzoli R, Yasothan U, Kirkpatrick P. Denosumab. Nat Rev Drug Discov. 2010;9:591–2. - PubMed
-
- Kanis JA, Johansson H, Oden A, McCloskey EV. Assessment of fracture risk. Eur J Radiol. 2009;71:392–7. - PubMed
-
- Hussar DA, Stevenson T. New drugs: denosumab, dienogest/estradiol valerate, and polidocanol. J Am Pharm Assoc. 2010;50:658–62. - PubMed
-
- Papaioannou A, Joseph L, Ioannidis G, et al. Risk factors associated with incident clinical vertebral and nonvertebral fractures in postmenopausal women: the Canadian Multicentre Osteoporosis Study (CaMos) Osteoporos Int. 2005;16:568–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

