The role of surface chemistry-induced cell characteristics on nonviral gene delivery to mouse fibroblasts
- PMID: 22967455
- PMCID: PMC3517526
- DOI: 10.1186/1754-1611-6-17
The role of surface chemistry-induced cell characteristics on nonviral gene delivery to mouse fibroblasts
Abstract
Background: Gene delivery approaches serve as a platform to modify gene expression of a cell population with applications including functional genomics, tissue engineering, and gene therapy. The delivery of exogenous genetic material via nonviral vectors has proven to be less toxic and to cause less of an immune response in comparison to viral vectors, but with decreased efficiency of gene transfer. Attempts have been made to improve nonviral gene transfer efficiency by modifying physicochemical properties of gene delivery vectors as well as developing new delivery techniques. In order to further improve and understand nonviral gene delivery, our approach focuses on the cell-material interface, since materials are known to modulate cell behavior, potentially rendering cells more responsive to nonviral gene transfer. In this study, self-assembled monolayers of alkanethiols on gold were employed as model biomaterial interfaces with varying surface chemistries. NIH/3T3 mouse fibroblasts were seeded on the modified surfaces and transfected using either lipid- or polymer- based complexing agents.
Results: Transfection was increased in cells on charged hydrophilic surfaces presenting carboxylic acid terminal functional groups, while cells on uncharged hydrophobic surfaces presenting methyl terminations demonstrated reduced transfection for both complexing agents. Surface-induced cellular characteristics that were hypothesized to affect nonviral gene transfer were subsequently investigated. Cells on charged hydrophilic surfaces presented higher cell densities, more cell spreading, more cells with ellipsoid morphologies, and increased quantities of focal adhesions and cytoskeleton features within cells, in contrast to cell on uncharged hydrophobic surfaces, and these cell behaviors were subsequently correlated to transfection characteristics.
Conclusions: Extracellular influences on nonviral gene delivery were investigated by evaluating the upregulation and downregulation of transgene expression as a function of the cell behaviors induced by changes in the cells' microenvronments. This study demonstrates that simple surface modifications can lead to changes in the efficiency of nonviral gene delivery. In addition, statistically significant differences in various surface-induced cell characteristics were statistically correlated to transfection trends in fibroblasts using both lipid and polymer mediated DNA delivery approaches. The correlations between the evaluated complexing agents and cell behaviors (cell density, spreading, shape, cytoskeleton, focal adhesions, and viability) suggest that polymer-mediated transfection is correlated to cell morphological traits while lipid-mediated transfection correlates to proliferative characteristics.
Figures

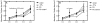

Similar articles
-
Surface- and Hydrogel-Mediated Delivery of Nucleic Acid Nanoparticles.Methods Mol Biol. 2019;1943:177-197. doi: 10.1007/978-1-4939-9092-4_12. Methods Mol Biol. 2019. PMID: 30838617
-
Optimizing nonviral-mediated transfection of human intervertebral disc chondrocytes.Spine J. 2008 Sep-Oct;8(5):796-803. doi: 10.1016/j.spinee.2007.05.010. Epub 2007 Jul 19. Spine J. 2008. PMID: 18023624
-
Surface-tethered DNA complexes for enhanced gene delivery.Bioconjug Chem. 2002 May-Jun;13(3):621-9. doi: 10.1021/bc015575f. Bioconjug Chem. 2002. PMID: 12009954
-
Nucleic acid delivery to mesenchymal stem cells: a review of nonviral methods and applications.J Biol Eng. 2019 Jan 18;13:7. doi: 10.1186/s13036-019-0140-0. eCollection 2019. J Biol Eng. 2019. PMID: 30675180 Free PMC article. Review.
-
Current development of nonviral-mediated gene transfer.Drug News Perspect. 2007 May;20(4):227-31. doi: 10.1358/dnp.2007.20.4.1103528. Drug News Perspect. 2007. PMID: 17637935 Review.
Cited by
-
Network analysis of endogenous gene expression profiles after polyethyleneimine-mediated DNA delivery.J Gene Med. 2013 Mar-Apr;15(3-4):142-54. doi: 10.1002/jgm.2704. J Gene Med. 2013. PMID: 23526566 Free PMC article.
-
Free Polyethylenimine Enhances Substrate-Mediated Gene Delivery on Titanium Substrates Modified With RGD-Functionalized Poly(acrylic acid) Brushes.Front Chem. 2019 Feb 7;7:51. doi: 10.3389/fchem.2019.00051. eCollection 2019. Front Chem. 2019. PMID: 30792979 Free PMC article.
-
Transfection in the third dimension.Integr Biol (Camb). 2013 Oct;5(10):1206-16. doi: 10.1039/c3ib40086g. Integr Biol (Camb). 2013. PMID: 23929354 Free PMC article.
-
Pathways Governing Polyethylenimine Polyplex Transfection in Microporous Annealed Particle Scaffolds.Bioconjug Chem. 2019 Feb 20;30(2):476-486. doi: 10.1021/acs.bioconjchem.8b00696. Epub 2018 Dec 18. Bioconjug Chem. 2019. PMID: 30513197 Free PMC article.
-
Droplet microarrays for cell culture: effect of surface properties and nanoliter culture volume on global transcriptomic landscape.Mater Today Bio. 2021 Apr 30;11:100112. doi: 10.1016/j.mtbio.2021.100112. eCollection 2021 Jun. Mater Today Bio. 2021. PMID: 34124640 Free PMC article.
References
LinkOut - more resources
Full Text Sources

