Gene location and DNA density determine transcription factor distributions in Escherichia coli
- PMID: 22968444
- PMCID: PMC3472691
- DOI: 10.1038/msb.2012.42
Gene location and DNA density determine transcription factor distributions in Escherichia coli
Abstract
The diffusion coefficient of the transcription factor LacI within living Escherichia coli has been measured directly by in vivo tracking to be D = 0.4 μm(2)/s. At this rate, simple models of diffusion lead to the expectation that LacI and other proteins will rapidly homogenize throughout the cell. Here, we test this expectation of spatial homogeneity by single-molecule visualization of LacI molecules non-specifically bound to DNA in fixed cells. Contrary to expectation, we find that the distribution depends on the spatial location of its encoding gene. We demonstrate that the spatial distribution of LacI is also determined by the local state of DNA compaction, and that E. coli can dynamically redistribute proteins by modifying the state of its nucleoid. Finally, we show that LacI inhomogeneity increases the strength with which targets located proximally to the LacI gene are regulated. We propose a model for intranucleoid diffusion that can reconcile these results with previous measurements of LacI diffusion, and we discuss the implications of these findings for gene regulation in bacteria and eukaryotes.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


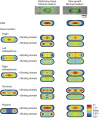
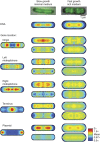
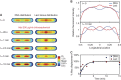
 . The equivalent result for LacI42-Venus is shown in Supplementary Figure 6. Source data is available for this figure in the Supplementary Information.
. The equivalent result for LacI42-Venus is shown in Supplementary Figure 6. Source data is available for this figure in the Supplementary Information.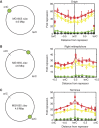

References
-
- Ahmed S, Brickner JH (2007) Regulation and epigenetic control of transcription at the nuclear periphery. Trends Genet 23: 396–402 - PubMed
-
- Berg OG, Winter RB, von Hippel PH (1981) Diffusion-driven mechanisms of protein translocation on nucleic acids. 1. Models and theory. Biochemistry 20: 6929–6948 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

