Gene therapy for adenosine deaminase-deficient severe combined immune deficiency: clinical comparison of retroviral vectors and treatment plans
- PMID: 22968453
- PMCID: PMC3488882
- DOI: 10.1182/blood-2012-02-400937
Gene therapy for adenosine deaminase-deficient severe combined immune deficiency: clinical comparison of retroviral vectors and treatment plans
Abstract
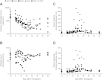
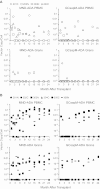
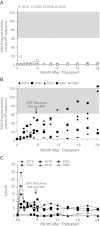
We conducted a gene therapy trial in 10 patients with adenosine deaminase (ADA)-deficient severe combined immunodeficiency using 2 slightly different retroviral vectors for the transduction of patients' bone marrow CD34(+) cells. Four subjects were treated without pretransplantation cytoreduction and remained on ADA enzyme-replacement therapy (ERT) throughout the procedure. Only transient (months), low-level (< 0.01%) gene marking was observed in PBMCs of 2 older subjects (15 and 20 years of age), whereas some gene marking of PBMC has persisted for the past 9 years in 2 younger subjects (4 and 6 years). Six additional subjects were treated using the same gene transfer protocol, but after withdrawal of ERT and administration of low-dose busulfan (65-90 mg/m(2)). Three of these remain well, off ERT (5, 4, and 3 years postprocedure), with gene marking in PBMC of 1%-10%, and ADA enzyme expression in PBMC near or in the normal range. Two subjects were restarted on ERT because of poor gene marking and immune recovery, and one had a subsequent allogeneic hematopoietic stem cell transplantation. These studies directly demonstrate the importance of providing nonmyeloablative pretransplantation conditioning to achieve therapeutic benefits with gene therapy for ADA-deficient severe combined immunodeficiency.
Figures
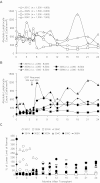
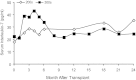
Comment in
-
Gene therapy for ADA-SCID: defining the factors for successful outcome.Blood. 2012 Nov 1;120(18):3628-9. doi: 10.1182/blood-2012-08-446559. Blood. 2012. PMID: 23118212 No abstract available.
References
-
- Giblett ER, Anderson JE, Cohen F, Pollara B, Meuwissen HJ. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972;2(7786):1067–1069. - PubMed
-
- Hershfield M, Mitchell B. Immunodeficiency diseases caused by adenosine deaminase deficiency and purine nucleotide phosphorylase deficiency. In: Scriver CS, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 7th Ed. New York, NY: McGraw-Hill Inc; 1995. pp. 1725–1768.
-
- Hirschhorn R. Overview of biochemical abnormalities and molecular genetics of adenosine deaminase deficiency. Pediatr Res. 1993;33(1 Suppl):S35–41. - PubMed
-
- Parkman R, Gelfand EW, Rosen FS, Sanderson A, Hirschhorn R. Severe combined immunodeficiency and adenosine deaminase deficiency. N Engl J Med. 1975;292(14):714–719. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

