Effect of tranexamic acid on mortality in patients with traumatic bleeding: prespecified analysis of data from randomised controlled trial
- PMID: 22968527
- PMCID: PMC3439642
- DOI: 10.1136/bmj.e5839
Effect of tranexamic acid on mortality in patients with traumatic bleeding: prespecified analysis of data from randomised controlled trial
Abstract
Objectives: To examine whether the effect of tranexamic acid on the risk of death and thrombotic events in patients with traumatic bleeding varies according to baseline risk of death. To assess the extent to which current protocols for treatment with tranexamic acid maximise benefits to patients.
Design: Prespecified stratified analysis of data from an international multicentre randomised controlled trial (the CRASH-2 trial) with an estimation of the proportion of premature deaths that could potentially be averted through the administration of tranexamic acid.
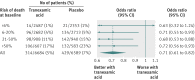
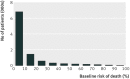
Participants: 13,273 trauma patients in the CRASH-2 trial who were treated with tranexamic acid or placebo within three hours of injury and trauma patients enrolled in UK Trauma and Audit Research Network, stratified by risk of death at baseline (<6%, 6-20%, 21-50%, >50%).
Intervention: Tranexamic acid (1 g over 10 minutes followed by 1 g over eight hours) or matching placebo.
Main outcome measure: Odds ratios and 95% confidence intervals for death in hospital within four weeks of injury, deaths from bleeding, and fatal and non-fatal thrombotic events associated with the use of tranexamic acid according to baseline risk of death. Unless there was strong evidence against the null hypothesis of homogeneity of effects (P<0.001), the overall odds ratio was used as the most reliable guide to the odds ratios in all strata.
Results: Tranexamic acid was associated with a significant reduction in all cause mortality and deaths from bleeding. In each stratum of baseline risk, there were fewer deaths among patients treated with tranexamic acid. There was no evidence of heterogeneity in the effect of tranexamic acid on all cause mortality (P=0.96 for interaction) or deaths from bleeding (P=0.98) by baseline risk of death. In those treated with tranexamic acid there was a significant reduction in the odds of fatal and non-fatal thrombotic events (odds ratio 0.69, 95% confidence interval 0.53 to 0.89; P=0.005) and a significant reduction in arterial thrombotic events (0.58, 0.40 to 0.83; P=0.003) but no significant reduction in venous thrombotic events (0.83, 0.59 to 1.17; P=0.295). There was no evidence of heterogeneity in the effect of tranexamic acid on the risk of thrombotic events (P=0.74). If the effect of tranexamic acid is assumed to be the same in all risk strata (<6%, 6-20%, 21-50%, >50% risk of death at baseline), the percentage of deaths that could be averted by administration of tranexamic acid within three hours of injury in each group is 17%, 36%, 30%, and 17%, respectively.
Conclusions: Tranexamic acid can be administered safely to a wide spectrum of patients with traumatic bleeding and should not be restricted to the most severely injured.
Trial registration: ISRCTN86750102.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures



References
-
- CRASH-2 Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23-32. - PubMed
-
- CRASH-2 collaborators. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 2011;377:1096-101. - PubMed
-
- TXA implementation pages—how to do it. www2.le.ac.uk/departments/cardiovascular-sciences/research/population-re....
-
- Committee on Tactical Combat Casualty Care. Tranexamic acid (TXA) in tactical combat casualty care. Guideline revision recommendation. 2011. www.medicalsci.com/files/tranexamic_acid__txa__in_tactical_combat_casual....
-
- Luz L, Sankarankutty A, Passos E, Rizoli S, Fraga G, Nascimento Jr B. Tranexamic acid for traumatic hemorrhage. Rev Col Bras Cir. 2012;39:77-80. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
