Miniaturized transfer models to predict the precipitation of poorly soluble weak bases upon entry into the small intestine
- PMID: 22968547
- PMCID: PMC3513478
- DOI: 10.1208/s12249-012-9851-y
Miniaturized transfer models to predict the precipitation of poorly soluble weak bases upon entry into the small intestine
Abstract
For poorly soluble weak bases, the possibility of drug precipitation upon entry into the small intestine may affect the amount of drug available for uptake through the intestinal mucosa. A few years ago, a transfer model was introduced which has been developed to simulate the transfer of a dissolved drug out of the stomach into the small intestine. However, this setup requires the use of clinically relevant doses of the drug, which are typically not available in the early stages of formulation development. The present series of tests was performed to check whether it is possible to create a miniaturized but physiologically relevant transfer model that can be applied in the early formulation development. Experiments were performed with two miniaturized setups: a 96-well plate model and a mini-paddle transfer system. Itraconazole and tamoxifen were used as model drugs. An appropriate amount of each drug formulation was dissolved in simulated gastric fluid and then transferred into an acceptor phase consisting of fasted/fed state simulated small intestinal fluid. The amount of drug dissolved in the acceptor phase was monitored over a period of 4 h. Results from both setups were very similar. The tamoxifen preformulation did not precipitate, whereas the itraconazole formulation precipitated to the same extent in both setups. Due to the possibility of generating physiologically relevant results but using smaller sample sizes and smaller volumes of media, both miniaturized transfer systems offer various advantages in terms of substance and analytical and material cost savings when evaluating the precipitation potential of poorly soluble weakly basic drug candidates.
Figures

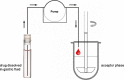
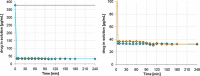



Similar articles
-
Predicting the precipitation of poorly soluble weak bases upon entry in the small intestine.J Pharm Pharmacol. 2004 Jan;56(1):43-51. doi: 10.1211/0022357022511. J Pharm Pharmacol. 2004. PMID: 14980000
-
Precipitation in the small intestine may play a more important role in the in vivo performance of poorly soluble weak bases in the fasted state: case example nelfinavir.Eur J Pharm Biopharm. 2011 Oct;79(2):349-56. doi: 10.1016/j.ejpb.2011.04.005. Epub 2011 Apr 18. Eur J Pharm Biopharm. 2011. PMID: 21527341 Clinical Trial.
-
Transfer Behavior of the Weakly Acidic BCS Class II Drug Valsartan from the Stomach to the Small Intestine During Fasted and Fed States.AAPS PharmSciTech. 2018 Jul;19(5):2213-2225. doi: 10.1208/s12249-018-1028-x. Epub 2018 May 7. AAPS PharmSciTech. 2018. PMID: 29736887
-
In vitro methods to assess drug precipitation in the fasted small intestine - a PEARRL review.J Pharm Pharmacol. 2019 Apr;71(4):536-556. doi: 10.1111/jphp.12951. Epub 2018 Jun 28. J Pharm Pharmacol. 2019. PMID: 29956338 Review.
-
In vitro-in vivo correlations for lipophilic, poorly water-soluble drugs.Eur J Pharm Sci. 2000 Oct;11 Suppl 2:S73-80. doi: 10.1016/s0928-0987(00)00181-0. Eur J Pharm Sci. 2000. PMID: 11033429 Review.
Cited by
-
Parameterization of Physiologically Based Biopharmaceutics Models: Workshop Summary Report.Mol Pharm. 2024 Aug 5;21(8):3697-3731. doi: 10.1021/acs.molpharmaceut.4c00526. Epub 2024 Jun 30. Mol Pharm. 2024. PMID: 38946085 Free PMC article. Review.
-
Development and Application of a Dissolution-Transfer-Partitioning System (DTPS) for Biopharmaceutical Drug Characterization.Pharmaceutics. 2023 Mar 26;15(4):1069. doi: 10.3390/pharmaceutics15041069. Pharmaceutics. 2023. PMID: 37111555 Free PMC article.
-
"Development of Fixed Dose Combination Products" Workshop Report: Considerations of Gastrointestinal Physiology and Overall Development Strategy.AAPS J. 2019 Jun 6;21(4):75. doi: 10.1208/s12248-019-0346-6. AAPS J. 2019. PMID: 31172358
References
-
- Heikkila T, Karjalainen M, Ojala K, Partola K, Lammert F, Augustijns P, et al. Equilibrium drug solubility measurements in 96-well plates reveal similar drug solubilities in phosphate buffer pH 6.8 and human intestinal fluid. Int J Pharm. 2011;405:132–136. doi: 10.1016/j.ijpharm.2010.12.007. - DOI - PubMed
-
- Buchanan NL, Buchanan CM. High throughput screening method for determination of equilibrium drug solubility. In: Edgar KJ, Heinze T, Buchanan CM, editors. Polysaccharide materials: performance by design. Washington, DC: American Chemical Society; 2009. pp. 65–80.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

