Miniaturized transfer models to predict the precipitation of poorly soluble weak bases upon entry into the small intestine
- PMID: 22968547
- PMCID: PMC3513478
- DOI: 10.1208/s12249-012-9851-y
Miniaturized transfer models to predict the precipitation of poorly soluble weak bases upon entry into the small intestine
Abstract
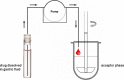
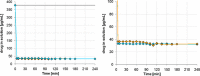
For poorly soluble weak bases, the possibility of drug precipitation upon entry into the small intestine may affect the amount of drug available for uptake through the intestinal mucosa. A few years ago, a transfer model was introduced which has been developed to simulate the transfer of a dissolved drug out of the stomach into the small intestine. However, this setup requires the use of clinically relevant doses of the drug, which are typically not available in the early stages of formulation development. The present series of tests was performed to check whether it is possible to create a miniaturized but physiologically relevant transfer model that can be applied in the early formulation development. Experiments were performed with two miniaturized setups: a 96-well plate model and a mini-paddle transfer system. Itraconazole and tamoxifen were used as model drugs. An appropriate amount of each drug formulation was dissolved in simulated gastric fluid and then transferred into an acceptor phase consisting of fasted/fed state simulated small intestinal fluid. The amount of drug dissolved in the acceptor phase was monitored over a period of 4 h. Results from both setups were very similar. The tamoxifen preformulation did not precipitate, whereas the itraconazole formulation precipitated to the same extent in both setups. Due to the possibility of generating physiologically relevant results but using smaller sample sizes and smaller volumes of media, both miniaturized transfer systems offer various advantages in terms of substance and analytical and material cost savings when evaluating the precipitation potential of poorly soluble weakly basic drug candidates.
Figures




References
-
- Heikkila T, Karjalainen M, Ojala K, Partola K, Lammert F, Augustijns P, et al. Equilibrium drug solubility measurements in 96-well plates reveal similar drug solubilities in phosphate buffer pH 6.8 and human intestinal fluid. Int J Pharm. 2011;405:132–136. doi: 10.1016/j.ijpharm.2010.12.007. - DOI - PubMed
-
- Buchanan NL, Buchanan CM. High throughput screening method for determination of equilibrium drug solubility. In: Edgar KJ, Heinze T, Buchanan CM, editors. Polysaccharide materials: performance by design. Washington, DC: American Chemical Society; 2009. pp. 65–80.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

