Metformin in non-diabetic patients presenting with ST elevation myocardial infarction: rationale and design of the glycometabolic intervention as adjunct to primary percutaneous intervention in ST elevation myocardial infarction (GIPS)-III trial
- PMID: 22968678
- PMCID: PMC3464381
- DOI: 10.1007/s10557-012-6413-1
Metformin in non-diabetic patients presenting with ST elevation myocardial infarction: rationale and design of the glycometabolic intervention as adjunct to primary percutaneous intervention in ST elevation myocardial infarction (GIPS)-III trial
Abstract
Background: Left ventricular dysfunction and the development of heart failure is a frequent and serious complication of myocardial infarction. Recent animal experimental studies suggested that metformin treatment reduces myocardial injury and preserves cardiac function in non-diabetic rats after experimental myocardial infarction. We will study the efficacy of metformin with the aim to preserve left ventricular ejection fraction in non-diabetic patients presenting with ST elevation myocardial infarction (STEMI).
Methods: The Glycometabolic Intervention as adjunct to Primary percutaneous intervention in ST elevation myocardial infarction (GIPS)-III trial is a prospective, single center, double blind, randomized, placebo-controlled trial. Three-hundred-and-fifty patients, without diabetes, requiring primary percutaneous coronary intervention (PCI) for STEMI will be randomized to metformin 500 mg twice daily or placebo treatment and will undergo magnetic resonance imaging (MRI) after 4 months. Major exclusion criteria were prior myocardial infarction and severe renal dysfunction. The primary efficacy parameter is left ventricular ejection fraction 4 months after randomization. Secondary and tertiary efficacy parameters include major adverse cardiac events, new onset diabetes and glycometabolic parameters, and echocardiographic diastolic function. Safety parameters include renal function deterioration and lactic acidosis.
Conclusions: The GIPS-III trial will evaluate the efficacy of metformin treatment to preserve left ventricular ejection fraction in STEMI patients without diabetes.
Trial registration: ClinicalTrials.gov NCT01217307.
Figures
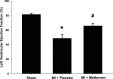


References
-
- Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. - PubMed
-
- Ryden L, Standl E, Bartnik M, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehm124. - DOI - PubMed
-
- Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L, et al. Prognostic implications of glucose-lowering treatment in patients with acute myocardial infarction and diabetes: experiences from an extended follow-up of the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) 2 Study. Diabetologia. 2011;54:1308–1317. doi: 10.1007/s00125-011-2084-x. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

