High-fat or ethinyl-oestradiol intake during pregnancy increases mammary cancer risk in several generations of offspring
- PMID: 22968699
- PMCID: PMC3570979
- DOI: 10.1038/ncomms2058
High-fat or ethinyl-oestradiol intake during pregnancy increases mammary cancer risk in several generations of offspring
Abstract
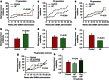
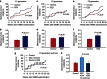
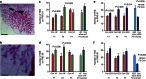
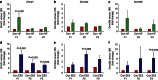
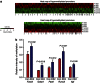
Maternal exposures to environmental factors during pregnancy influence the risk of many chronic adult-onset diseases in the offspring. Here we investigate whether feeding pregnant rats a high-fat (HF)- or ethinyl-oestradiol (EE2)-supplemented diet affects carcinogen-induced mammary cancer risk in daughters, granddaughters and great-granddaughters. We show that mammary tumourigenesis is higher in daughters and granddaughters of HF rat dams and in daughters and great-granddaughters of EE2 rat dams. Outcross experiments suggest that the increase in mammary cancer risk is transmitted to HF granddaughters equally through the female or male germ lines, but it is only transmitted to EE2 granddaughters through the female germ line. The effects of maternal EE2 exposure on offspring's mammary cancer risk are associated with changes in the DNA methylation machinery and methylation patterns in mammary tissue of all three EE2 generations. We conclude that dietary and oestrogenic exposures in pregnancy increase breast cancer risk in multiple generations of offspring, possibly through epigenetic means.
Conflict of interest statement
Dr Hilakivi-Clarke has been retained as a consultant in litigation on behalf of DES daughters claiming breast cancer injury due to DES exposure. The remaining authors declare no competing financial interests
Figures
References
-
- Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet 358, 1389–1399 (2001). - PubMed
-
- Antoniou A. C. & Easton D. F. Models of genetic susceptibility to breast cancer. Oncogene 25, 5898–5905 (2006). - PubMed
-
- Oldenburg R. A., Meijers-Heijboer H., Cornelisse C. J. & Devilee P. Genetic susceptibility for breast cancer: how many more genes to be found? Crit. Rev. Oncol. Hematol. 63, 125–149 (2007). - PubMed
-
- Kawaguchi H. et al. Effects of exposure period and dose of diethylstilbestrol on pregnancy in rats. J. Vet. Med. Sci. 71, 1309–1315 (2009). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R21 GM085665/GM/NIGMS NIH HHS/United States
- U54 CA149147/CA/NCI NIH HHS/United States
- U54CA113001/CA/NCI NIH HHS/United States
- U54CA100970/CA/NCI NIH HHS/United States
- U24 CA160036/CA/NCI NIH HHS/United States
- R01 CA164384/CA/NCI NIH HHS/United States
- P30 CA054174/CA/NCI NIH HHS/United States
- R01ES017594/ES/NIEHS NIH HHS/United States
- R03 CA150040/CA/NCI NIH HHS/United States
- P30CA054174/CA/NCI NIH HHS/United States
- R01CA069065/CA/NCI NIH HHS/United States
- R21GM085665/GM/NIGMS NIH HHS/United States
- U54CA149147/CA/NCI NIH HHS/United States
- P30CA051668/CA/NCI NIH HHS/United States
- R01 CA069065/CA/NCI NIH HHS/United States
- R01 ES017594/ES/NIEHS NIH HHS/United States
- U54 CA100970/CA/NCI NIH HHS/United States
- CA160036/CA/NCI NIH HHS/United States
- R03CA150040/CA/NCI NIH HHS/United States
- T32 CA009686/CA/NCI NIH HHS/United States
- U54 CA113001/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

