Molecular, cellular, and structural mechanisms of cocaine addiction: a key role for microRNAs
- PMID: 22968819
- PMCID: PMC3521966
- DOI: 10.1038/npp.2012.120
Molecular, cellular, and structural mechanisms of cocaine addiction: a key role for microRNAs
Abstract
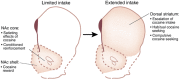
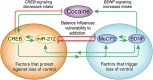
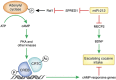
The rewarding properties of cocaine play a key role in establishing and maintaining the drug-taking habit. However, as exposure to cocaine increases, drug use can transition from controlled to compulsive. Importantly, very little is known about the neurobiological mechanisms that control this switch in drug use that defines addiction. MicroRNAs (miRNAs) are small non-protein coding RNA transcripts that can regulate the expression of messenger RNAs that code for proteins. Because of their highly pleiotropic nature, each miRNA has the potential to regulate hundreds or even thousands of protein-coding RNA transcripts. This property of miRNAs has generated considerable interest in their potential involvement in complex psychiatric disorders such as addiction, as each miRNA could potentially influence the many different molecular and cellular adaptations that arise in response to drug use that are hypothesized to drive the emergence of addiction. Here, we review recent evidence supporting a key role for miRNAs in the ventral striatum in regulating the rewarding and reinforcing properties of cocaine in animals with limited exposure to the drug. Moreover, we discuss evidence suggesting that miRNAs in the dorsal striatum control the escalation of drug intake in rats with extended cocaine access. These findings highlight the central role for miRNAs in drug-induced neuroplasticity in brain reward systems that drive the emergence of compulsive-like drug use in animals, and suggest that a better understanding of how miRNAs control drug intake will provide new insights into the neurobiology of drug addiction.
Figures


References
-
- Abuhatzira L, Makedonski K, Kaufman Y, Razin A, Shemer R. MeCP2 deficiency in the brain decreases BDNF levels by REST/CoREST-mediated repression and increases TRKB production. Epigenetics. 2007;2:214–222. - PubMed
-
- Acar M, Becskei A, van Oudenaarden A. Enhancement of cellular memory by reducing stochastic transitions. Nature. 2005;435:228–232. - PubMed
-
- Ahmed SH. Imbalance between drug and non-drug reward availability: a major risk factor for addiction. Eur J Pharmacol. 2005;526:9–20. - PubMed
-
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. - PubMed
-
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

