Silyl ketene imines: highly versatile nucleophiles for catalytic, asymmetric synthesis
- PMID: 22968901
- PMCID: PMC3532842
- DOI: 10.1002/anie.201202139
Silyl ketene imines: highly versatile nucleophiles for catalytic, asymmetric synthesis
Abstract
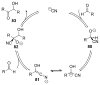
This Minireview provides an overview on the development of silyl ketene imines and their recent applications in catalytic, enantioselective reactions. The unique structure of the ketene imine allows a diverse range of reactivity patterns and provides solutions to existing challenges in the enantioselective construction of quaternary stereogenic carbon centers and cross-benzoin adducts. A variety of reactions for which silyl ketene imines have been applied are presented with an overall goal of inspiring new uses for these underutilized nucleophiles.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures





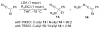











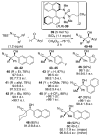
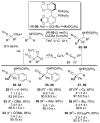



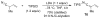


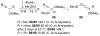



References
-
- Patai S. The Chemistry of Ketenes, Allenes and Related Compounds. Wiley; New York: 1980.
- Schuster HF, Coppola GM. Allenes in Organic Synthesis. Wiley; New York: 1984.
- Tidwell TT. Ketenes. Wiley; New York: 1995.
-
- Brownbridge P. Synthesis. 1983:1–28.
- Brownbridge P. Synthesis. 1983:85–104.
- Kita Y, Tamura O, Tamura Y. J Synth Org Chem Jpn. 1986;44:1118–1133.
- Kobayashi S, Manabe K, Ishitani H, Matsuo J-I. In: Science of Synthesis Organometallics: Silicon Compounds. Fleming I, Ley SV, editors. Vol. 4. Georg Thieme; Stuttgart: 2004. pp. 316–369.
-
- Narasaka K, Soai K, Mukaiyam T. Chem Lett. 1974:1223–1224.
- Oare DA, Heathcock CH. Top Stereochem. 1991;20:87–170.
- Oare DA, Heathcock CH. Top Stereochem. 1989;19:227–407.
-
- Mukaiyama T. Org React. 1982;28:203–331.
- Mukaiyama T, Banno K, Narasaka K. J Am Chem Soc. 1974;96:7503–7509.
- Mukaiyama T, Narasaka K, Banno K. Chem Lett. 1973:1011–1014.
- Mahrwald R. Modern Aldol Reactions. Wiley-VCH; Weinheim: 2004.
-
- Denissova I, Barriault L. Tetrahedron. 2003;59:10105–10146.
- Douglas CJ, Overman LE. Proc Natl Acad Sci USA. 2004;101:5363–5367. - PMC - PubMed
- Trost BM, Jiang C. Synthesis. 2006:369–396.
- Das JP, Marek I. Chem Commun. 2011;47:4593–4623. - PubMed
- Christoffers J, Baro A. Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis. Wiley-VCH; Weinheim: 2005.
Grants and funding
LinkOut - more resources
Full Text Sources

