Obesity-associated dysregulation of calpastatin and MMP-15 in adipose-derived stromal cells results in their enhanced invasion
- PMID: 22969001
- PMCID: PMC5973795
- DOI: 10.1002/stem.1229
Obesity-associated dysregulation of calpastatin and MMP-15 in adipose-derived stromal cells results in their enhanced invasion
Abstract
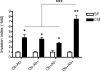
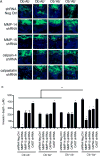
Adipose tissue maintains a subpopulation of cells, referred to as adipose-derived stromal/stem cells (ASCs), which have been associated with increased breast cancer tumorigenesis and metastasis. For ASCs to affect breast cancer cells, it is necessary to delineate how they mobilize and home to cancer cells, which requires mobilization and invasion through extracellular matrix barriers. In this study, ASCs were separated into four different categories based on the donor's obesity status and depot site of origin. ASCs isolated from the subcutaneous abdominal adipose tissue of obese patients (Ob(+)Ab(+)) demonstrated increased invasion through Matrigel as well as a chick chorioallantoic membrane, a type I collagen-rich extracellular matrix barrier. Detailed mRNA and protein analyses revealed that calpain-4, calpastatin, and MMP-15 were associated with increased invasion, and the silencing of each protease or protease inhibitor confirmed their role in ASC invasion. Thus, the data indicate that both the donor's obesity status and depot site of origin distinguishes the properties of subcutaneous-derived ASCs with respect to enhanced invasion and this is associated with the dysregulation of calpain-4, calpastatin, and MMP-15.
Copyright © 2012 AlphaMed Press.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

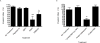

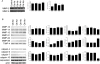
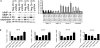
Similar articles
-
Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers.Breast Cancer Res. 2015 Aug 19;17(1):112. doi: 10.1186/s13058-015-0622-z. Breast Cancer Res. 2015. PMID: 26286584 Free PMC article.
-
Interaction with adipocyte stromal cells induces breast cancer malignancy via S100A7 upregulation in breast cancer microenvironment.Breast Cancer Res. 2017 Jun 19;19(1):70. doi: 10.1186/s13058-017-0863-0. Breast Cancer Res. 2017. PMID: 28629450 Free PMC article.
-
Obesity associated alterations in the biology of adipose stem cells mediate enhanced tumorigenesis by estrogen dependent pathways.Breast Cancer Res. 2013;15(5):R102. doi: 10.1186/bcr3569. Breast Cancer Res. 2013. PMID: 24176089 Free PMC article.
-
The effect of obesity on adipose-derived stromal cells and adipose tissue and their impact on cancer.Cancer Metastasis Rev. 2022 Sep;41(3):549-573. doi: 10.1007/s10555-022-10063-1. Epub 2022 Aug 24. Cancer Metastasis Rev. 2022. PMID: 35999486 Review.
-
Adipose stem cells in obesity: challenges and opportunities.Biosci Rep. 2020 Jun 26;40(6):BSR20194076. doi: 10.1042/BSR20194076. Biosci Rep. 2020. PMID: 32452515 Free PMC article. Review.
Cited by
-
Is There A Causal Relationship between Childhood Obesity and Acute Lymphoblastic Leukemia? A Review.Cancers (Basel). 2020 Oct 22;12(11):3082. doi: 10.3390/cancers12113082. Cancers (Basel). 2020. PMID: 33105727 Free PMC article. Review.
-
Obesity Enhances the Conversion of Adipose-Derived Stromal/Stem Cells into Carcinoma-Associated Fibroblast Leading to Cancer Cell Proliferation and Progression to an Invasive Phenotype.Stem Cells Int. 2017;2017:9216502. doi: 10.1155/2017/9216502. Epub 2017 Dec 17. Stem Cells Int. 2017. PMID: 29527228 Free PMC article.
-
Leptin produced by obesity-altered adipose stem cells promotes metastasis but not tumorigenesis of triple-negative breast cancer in orthotopic xenograft and patient-derived xenograft models.Breast Cancer Res. 2019 May 22;21(1):67. doi: 10.1186/s13058-019-1153-9. Breast Cancer Res. 2019. PMID: 31118047 Free PMC article.
-
Human Adipose-Derived Hydrogel Characterization Based on In Vitro ASC Biocompatibility and Differentiation.Stem Cells Int. 2019 Dec 27;2019:9276398. doi: 10.1155/2019/9276398. eCollection 2019. Stem Cells Int. 2019. PMID: 32082388 Free PMC article.
-
Human Obesity Attenuates Cardioprotection Conferred by Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells.J Cardiovasc Transl Res. 2023 Feb;16(1):221-232. doi: 10.1007/s12265-022-10279-0. Epub 2022 May 26. J Cardiovasc Transl Res. 2023. PMID: 35616881
References
-
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. - PubMed
-
- Healy LA, Ryan AM, Carroll P, et al. Metabolic syndrome, central obesity and insulin resistance are associated with adverse pathological features in postmenopausal breast cancer. Clin Oncol. 2010;22:281–288. - PubMed
-
- Petrelli JM, Calle EE, Rodriguez C, et al. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control. 2002;13:325–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

