The enzymes of biotin dependent CO₂ metabolism: what structures reveal about their reaction mechanisms
- PMID: 22969052
- PMCID: PMC3527699
- DOI: 10.1002/pro.2156
The enzymes of biotin dependent CO₂ metabolism: what structures reveal about their reaction mechanisms
Abstract
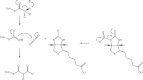
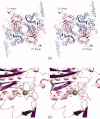
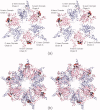
Biotin is the major cofactor involved in carbon dioxide metabolism. Indeed, biotin-dependent enzymes are ubiquitous in nature and are involved in a myriad of metabolic processes including fatty acid synthesis and gluconeogenesis. The cofactor, itself, is composed of a ureido ring, a tetrahydrothiophene ring, and a valeric acid side chain. It is the ureido ring that functions as the CO₂ carrier. A complete understanding of biotin-dependent enzymes is critically important for translational research in light of the fact that some of these enzymes serve as targets for anti-obesity agents, antibiotics, and herbicides. Prior to 1990, however, there was a dearth of information regarding the molecular architectures of biotin-dependent enzymes. In recent years there has been an explosion in the number of three-dimensional structures reported for these proteins. Here we review our current understanding of the structures and functions of biotin-dependent enzymes. In addition, we provide a critical analysis of what these structures have and have not revealed about biotin-dependent catalysis.
Copyright © 2012 The Protein Society.
Figures




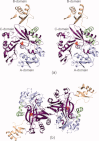
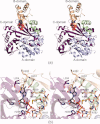


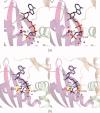




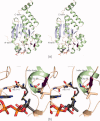



Similar articles
-
Functionally diverse biotin-dependent enzymes with oxaloacetate decarboxylase activity.Arch Biochem Biophys. 2014 Feb 15;544:75-86. doi: 10.1016/j.abb.2013.10.014. Epub 2013 Oct 30. Arch Biochem Biophys. 2014. PMID: 24184447 Review.
-
The biotin enzyme family: conserved structural motifs and domain rearrangements.Curr Protein Pept Sci. 2003 Jun;4(3):217-29. doi: 10.2174/1389203033487199. Curr Protein Pept Sci. 2003. PMID: 12769720 Review.
-
A substrate-induced biotin binding pocket in the carboxyltransferase domain of pyruvate carboxylase.J Biol Chem. 2013 Jul 5;288(27):19915-25. doi: 10.1074/jbc.M113.477828. Epub 2013 May 22. J Biol Chem. 2013. PMID: 23698000 Free PMC article.
-
Hybrid Structure of a Dynamic Single-Chain Carboxylase from Deinococcus radiodurans.Structure. 2016 Aug 2;24(8):1227-1236. doi: 10.1016/j.str.2016.06.001. Epub 2016 Jul 7. Structure. 2016. PMID: 27396827
-
Characterization of the carboxylate delivery module of transcarboxylase: following spontaneous decarboxylation of the 1.3S-CO2- subunit by NMR and FTIR spectroscopies.Biochemistry. 2002 Feb 19;41(7):2191-7. doi: 10.1021/bi0116442. Biochemistry. 2002. PMID: 11841210
Cited by
-
Discovery, structure, and function of filamentous 3-methylcrotonyl-CoA carboxylase.Structure. 2023 Jan 5;31(1):100-110.e4. doi: 10.1016/j.str.2022.11.015. Epub 2022 Dec 20. Structure. 2023. PMID: 36543169 Free PMC article.
-
Crystal structure of the 500-kDa yeast acetyl-CoA carboxylase holoenzyme dimer.Nature. 2015 Oct 29;526(7575):723-7. doi: 10.1038/nature15375. Epub 2015 Oct 12. Nature. 2015. PMID: 26458104 Free PMC article.
-
CryoEM reveals oligomeric isomers of a multienzyme complex and assembly mechanics.J Struct Biol X. 2023 Apr 8;7:100088. doi: 10.1016/j.yjsbx.2023.100088. eCollection 2023. J Struct Biol X. 2023. PMID: 37128595 Free PMC article.
-
The overlooked role of a biotin precursor for marine bacteria - desthiobiotin as an escape route for biotin auxotrophy.ISME J. 2022 Nov;16(11):2599-2609. doi: 10.1038/s41396-022-01304-w. Epub 2022 Aug 13. ISME J. 2022. PMID: 35963899 Free PMC article.
-
Effects of Betaine-Biotin-Chromium Supplementation and Concentrate to Roughage Ratio on Nutrient Utilization Efficiency in Thai Native Cattle.Animals (Basel). 2021 Sep 20;11(9):2747. doi: 10.3390/ani11092747. Animals (Basel). 2021. PMID: 34573713 Free PMC article.
References
-
- Kögl F, Tönnis B. Uber das Bios-Problem. Darstellung von Krystallisierten Biotin aus Eigelb. Z Physiol Chem. 1936;242:43–73.
-
- Eakin RE, Snell EE, Williams RJ. A constituent of raw egg white capable of inactivating biotin in vitro. J Biol Chem. 1940;136:801–802.
-
- du Vigneaud V. The structure of biotin. Science. 1942;96:455–461. - PubMed
-
- Lardy HA, Peanasky R. Metabolic functions of biotin. Physiol Rev. 1953;33:560–565. - PubMed
-
- Wakil SJ, Titchener EB, Gibson DM. Evidence for the participation of biotin in the enzymic synthesis of fatty acids. Biochim Biophys Acta. 1958;29:225–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

