Discovery of small-molecule inhibitors of the TLR1/TLR2 complex
- PMID: 22969053
- PMCID: PMC3510333
- DOI: 10.1002/anie.201204910
Discovery of small-molecule inhibitors of the TLR1/TLR2 complex
Abstract
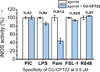
The protein complex of toll-like receptor 1 and 2 (TLR1/2) is an important regulator of innate immunity, and therefore provides an attractive target for the treatment of various immune disordres. Here we report a novel compound (CU-CPT22) that can compete with the synthetic triacylated lipoprotein (Pam3CSK4) binding to TLR1/2 with high inhibitory activity and specificity. Repression of downstream signaling from TNF-α and IL-1β has also been observed.
Figures
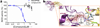

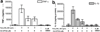

Similar articles
-
Phloretin as a Potent Natural TLR2/1 Inhibitor Suppresses TLR2-Induced Inflammation.Nutrients. 2018 Jul 5;10(7):868. doi: 10.3390/nu10070868. Nutrients. 2018. PMID: 29976865 Free PMC article.
-
The novel small-molecule antagonist MMG-11 preferentially inhibits TLR2/1 signaling.Biochem Pharmacol. 2020 Jan;171:113687. doi: 10.1016/j.bcp.2019.113687. Epub 2019 Nov 1. Biochem Pharmacol. 2020. PMID: 31678495
-
Vibrio cholerae porin OmpU mediates M1-polarization of macrophages/monocytes via TLR1/TLR2 activation.Immunobiology. 2015 Nov;220(11):1199-209. doi: 10.1016/j.imbio.2015.06.009. Epub 2015 Jun 5. Immunobiology. 2015. PMID: 26093918
-
Structure-based discovery of an immunomodulatory inhibitor of TLR1-TLR2 heterodimerization from a natural product-like database.Chem Commun (Camb). 2015 Jun 30;51(56):11178-81. doi: 10.1039/c5cc02728d. Chem Commun (Camb). 2015. PMID: 26051605
-
Black tea polyphenols-mediated in vivo cellular responses during carcinogenesis.Mini Rev Med Chem. 2010 Jun;10(6):492-505. doi: 10.2174/138955710791384063. Mini Rev Med Chem. 2010. PMID: 20370700 Review.
Cited by
-
The Race between Host Antiviral Innate Immunity and the Immune Evasion Strategies of Herpes Simplex Virus 1.Microbiol Mol Biol Rev. 2020 Sep 30;84(4):e00099-20. doi: 10.1128/MMBR.00099-20. Print 2020 Nov 18. Microbiol Mol Biol Rev. 2020. PMID: 32998978 Free PMC article. Review.
-
Amantadine attenuates sepsis-induced cognitive dysfunction possibly not through inhibiting toll-like receptor 2.J Mol Med (Berl). 2018 May;96(5):391-402. doi: 10.1007/s00109-018-1631-z. Epub 2018 Mar 3. J Mol Med (Berl). 2018. PMID: 29502203 Free PMC article.
-
Differential Activation of Innate Immune Pathways by Distinct Islet Amyloid Polypeptide (IAPP) Aggregates.J Biol Chem. 2016 Apr 22;291(17):8908-17. doi: 10.1074/jbc.M115.712455. Epub 2016 Jan 19. J Biol Chem. 2016. PMID: 26786104 Free PMC article.
-
TLR2/4 signaling pathway mediates sperm-induced inflammation in bovine endometrial epithelial cells in vitro.PLoS One. 2019 Apr 17;14(4):e0214516. doi: 10.1371/journal.pone.0214516. eCollection 2019. PLoS One. 2019. PMID: 30995239 Free PMC article.
-
Phloretin as a Potent Natural TLR2/1 Inhibitor Suppresses TLR2-Induced Inflammation.Nutrients. 2018 Jul 5;10(7):868. doi: 10.3390/nu10070868. Nutrients. 2018. PMID: 29976865 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

