Structure and location of the regulatory β subunits in the (αβγδ)4 phosphorylase kinase complex
- PMID: 22969083
- PMCID: PMC3481268
- DOI: 10.1074/jbc.M112.412874
Structure and location of the regulatory β subunits in the (αβγδ)4 phosphorylase kinase complex
Abstract
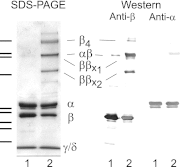
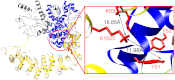
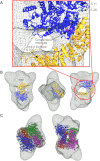
Phosphorylase kinase (PhK) is a hexadecameric (αβγδ)(4) complex that regulates glycogenolysis in skeletal muscle. Activity of the catalytic γ subunit is regulated by allosteric activators targeting the regulatory α, β, and δ subunits. Three-dimensional EM reconstructions of PhK show it to be two large (αβγδ)(2) lobes joined with D(2) symmetry through interconnecting bridges. The subunit composition of these bridges was unknown, although indirect evidence suggested the β subunits may be involved in their formation. We have used biochemical, biophysical, and computational approaches to not only address the quaternary structure of the β subunits within the PhK complex, i.e. whether they compose the bridges, but also their secondary and tertiary structures. The secondary structure of β was determined to be predominantly helical by comparing the CD spectrum of an αγδ subcomplex with that of the native (αβγδ)(4) complex. An atomic model displaying tertiary structure for the entire β subunit was constructed using chemical cross-linking, MS, threading, and ab initio approaches. Nearly all this model is covered by two templates corresponding to glycosyl hydrolase 15 family members and the A subunit of protein phosphatase 2A. Regarding the quaternary structure of the β subunits, they were directly determined to compose the four interconnecting bridges in the (αβγδ)(4) kinase core, because a β(4) subcomplex was observed through both chemical cross-linking and top-down MS of PhK. The predicted model of the β subunit was docked within the bridges of a cryoelectron microscopic density envelope of PhK utilizing known surface features of the subunit.
Figures

Similar articles
-
The structure of the large regulatory α subunit of phosphorylase kinase examined by modeling and hydrogen-deuterium exchange.Protein Sci. 2018 Feb;27(2):472-484. doi: 10.1002/pro.3339. Epub 2017 Nov 21. Protein Sci. 2018. PMID: 29098725 Free PMC article.
-
Evidence for the location of the allosteric activation switch in the multisubunit phosphorylase kinase complex from mass spectrometric identification of chemically crosslinked peptides.J Mol Biol. 2007 Feb 2;365(5):1429-45. doi: 10.1016/j.jmb.2006.10.061. Epub 2006 Oct 21. J Mol Biol. 2007. PMID: 17123541 Free PMC article.
-
Mass spectrometry reveals differences in stability and subunit interactions between activated and nonactivated conformers of the (αβγδ)4 phosphorylase kinase complex.Mol Cell Proteomics. 2012 Dec;11(12):1768-76. doi: 10.1074/mcp.M112.021394. Epub 2012 Sep 10. Mol Cell Proteomics. 2012. PMID: 22964223 Free PMC article.
-
Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure.Front Biosci. 1999 Sep 15;4:D618-41. doi: 10.2741/brushia. Front Biosci. 1999. PMID: 10487978 Review.
-
Substrate and inhibitor recognition of protein kinases: what is known about the catalytic subunit of phosphorylase kinase?Pharmacol Ther. 1999 May-Jun;82(2-3):143-55. doi: 10.1016/s0163-7258(98)00049-7. Pharmacol Ther. 1999. PMID: 10454193 Review.
Cited by
-
Structural characterization of the catalytic γ and regulatory β subunits of phosphorylase kinase in the context of the hexadecameric enzyme complex.Protein Sci. 2018 Feb;27(2):485-497. doi: 10.1002/pro.3340. Epub 2017 Nov 21. Protein Sci. 2018. PMID: 29098736 Free PMC article.
-
Architecture and activation of human muscle phosphorylase kinase.Nat Commun. 2024 Mar 28;15(1):2719. doi: 10.1038/s41467-024-47049-2. Nat Commun. 2024. PMID: 38548794 Free PMC article.
-
Mass Spectrometric Analysis of Surface-Exposed Regions in the Hexadecameric Phosphorylase Kinase Complex.Biochemistry. 2015 Nov 24;54(46):6887-95. doi: 10.1021/acs.biochem.5b00682. Epub 2015 Nov 13. Biochemistry. 2015. PMID: 26551836 Free PMC article.
-
Transcriptomic profile of leg muscle during early growth in chicken.PLoS One. 2017 Mar 14;12(3):e0173824. doi: 10.1371/journal.pone.0173824. eCollection 2017. PLoS One. 2017. PMID: 28291821 Free PMC article.
-
Activation of Phosphorylase Kinase by Physiological Temperature.Biochemistry. 2015 Dec 29;54(51):7524-30. doi: 10.1021/acs.biochem.5b01032. Epub 2015 Dec 14. Biochemistry. 2015. PMID: 26632861 Free PMC article.
References
-
- Brushia R. J., Walsh D. A. (1999) Phosphorylase kinase. The complexity of its regulation is reflected in the complexity of its structure. Front. Biosci. 4, D618–641 - PubMed
-
- Owen D. J., Noble M. E., Garman E. F., Papageorgiou A. C., Johnson L. N. (1995) Two structures of the catalytic domain of phosphorylase kinase. An active protein kinase complexed with substrate analogue and product. Structure 3, 467–482 - PubMed
-
- Dasgupta M., Honeycutt T., Blumenthal D. K. (1989) The γ-subunit of skeletal muscle phosphorylase kinase contains two noncontiguous domains that act in concert to bind calmodulin. J. Biol. Chem. 264, 17156–17163 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

