Disruption of P450-mediated vitamin E hydroxylase activities alters vitamin E status in tocopherol supplemented mice and reveals extra-hepatic vitamin E metabolism
- PMID: 22969154
- PMCID: PMC3494260
- DOI: 10.1194/jlr.M030734
Disruption of P450-mediated vitamin E hydroxylase activities alters vitamin E status in tocopherol supplemented mice and reveals extra-hepatic vitamin E metabolism
Abstract
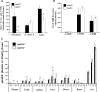
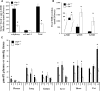
The widely conserved preferential accumulation of α-tocopherol (α-TOH) in tissues occurs, in part, from selective postabsorptive catabolism of non-α-TOH forms via the vitamin E-ω-oxidation pathway. We previously showed that global disruption of CYP4F14, the major but not the only mouse TOH-ω-hydroxylase, resulted in hyper-accumulation of γ-TOH in mice fed a soybean oil diet. In the current study, supplementation of Cyp4f14(-/-) mice with high levels of δ- and γ-TOH exacerbated tissue enrichment of these forms of vitamin E. However, at high dietary levels of TOH, mechanisms other than ω-hydroxylation dominate in resisting diet-induced accumulation of non-α-TOH. These include TOH metabolism via ω-1/ω-2 oxidation and fecal elimination of unmetabolized TOH. The ω-1 and ω-2 fecal metabolites of γ- and α-TOH were observed in human fecal material. Mice lacking all liver microsomal CYP activity due to disruption of cytochrome P450 reductase revealed the presence of extra-hepatic ω-, ω-1, and ω-2 TOH hydroxylase activities. TOH-ω-hydroxylase activity was exhibited by microsomes from mouse and human small intestine; murine activity was entirely due to CYP4F14. These findings shed new light on the role of TOH-ω-hydroxylase activity and other mechanisms in resisting diet-induced accumulation of tissue TOH and further characterize vitamin E metabolism in mice and humans.
Figures


Similar articles
-
Disruption of mouse cytochrome p450 4f14 (Cyp4f14 gene) causes severe perturbations in vitamin E metabolism.J Biol Chem. 2012 Jul 27;287(31):26077-86. doi: 10.1074/jbc.M112.373597. Epub 2012 Jun 4. J Biol Chem. 2012. PMID: 22665481 Free PMC article.
-
Common variants of cytochrome P450 4F2 exhibit altered vitamin E-{omega}-hydroxylase specific activity.J Nutr. 2010 Nov;140(11):1901-6. doi: 10.3945/jn.110.128579. Epub 2010 Sep 22. J Nutr. 2010. PMID: 20861217 Free PMC article.
-
Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status.J Biol Chem. 2002 Jul 12;277(28):25290-6. doi: 10.1074/jbc.M201466200. Epub 2002 May 7. J Biol Chem. 2002. PMID: 11997390
-
Discovery, characterization, and significance of the cytochrome P450 omega-hydroxylase pathway of vitamin E catabolism.Ann N Y Acad Sci. 2004 Dec;1031:13-21. doi: 10.1196/annals.1331.002. Ann N Y Acad Sci. 2004. PMID: 15753130 Review.
-
Regulation of xenobiotic metabolism, the only signaling function of alpha-tocopherol?Mol Nutr Food Res. 2010 May;54(5):661-8. doi: 10.1002/mnfr.200900440. Mol Nutr Food Res. 2010. PMID: 20169584 Review.
Cited by
-
Natural 6-hydroxy-chromanols and -chromenols: structural diversity, biosynthetic pathways and health implications.RSC Adv. 2018 Jan 26;8(9):4803-4841. doi: 10.1039/c7ra11819h. eCollection 2018 Jan 24. RSC Adv. 2018. PMID: 35539527 Free PMC article. Review.
-
Complexity of vitamin E metabolism.World J Biol Chem. 2016 Feb 26;7(1):14-43. doi: 10.4331/wjbc.v7.i1.14. World J Biol Chem. 2016. PMID: 26981194 Free PMC article. Review.
-
Pharmacokinetics of vitamin dosage forms: A complete overview.Food Sci Nutr. 2023 Nov 9;12(1):48-83. doi: 10.1002/fsn3.3787. eCollection 2024 Jan. Food Sci Nutr. 2023. PMID: 38268871 Free PMC article. Review.
-
Are Vitamin E Supplementation Beneficial for Female Gynaecology Health and Diseases?Molecules. 2022 Mar 15;27(6):1896. doi: 10.3390/molecules27061896. Molecules. 2022. PMID: 35335260 Free PMC article. Review.
-
Exploring the link between fat-soluble vitamins and aging-associated immune system status: a literature review.Immun Ageing. 2025 Feb 17;22(1):8. doi: 10.1186/s12979-025-00501-3. Immun Ageing. 2025. PMID: 39962579 Free PMC article. Review.
References
-
- Bieri J. G., Evarts R. P. 1973. Tocopherols and fatty acids in American diets. The recommended allowance for vitamin E. J. Am. Diet. Assoc. 62: 147–151 - PubMed
-
- Dial S., Eitenmiller R. R.1995. Tocopherols and tocotrienols in key foods in the U.S. diet. In Nutrition, Lipids, Health, and Disease. A. S. H. Ong, E. Niki, and L. Packer, editors. AOCS Press, Champaign, IL. 327–342.
-
- Ford E. S., Schleicher R. L., Mokdad A. H., Ajani U. A., Liu S. M. 2006. Distribution of serum concentrations of alpha-tocopherol and gamma-tocopherol in the US population. Am. J. Clin. Nutr. 84: 375–383 - PubMed
-
- Yoshida Y., Niki E., Noguchi N. 2003. Comparative study on the action of tocopherols and tocotrienols as antioxidant: chemical and physical effects. Chem. Phys. Lipids. 123: 63–75 - PubMed
-
- Saito Y., Yoshida Y., Akazawa T., Takahashi K., Niki E. 2003. Cell death caused by selenium deficiency and protective effect of antioxidants. J. Biol. Chem. 278: 39428–39434 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

