Adeno-associated virus mediated delivery of Tregitope 167 ameliorates experimental colitis
- PMID: 22969191
- PMCID: PMC3436043
- DOI: 10.3748/wjg.v18.i32.4288
Adeno-associated virus mediated delivery of Tregitope 167 ameliorates experimental colitis
Abstract
Aim: To explore the anti-inflammatory potential of adeno-associated virus-mediated delivery of Tregitope 167 in an experimental colitis model.
Methods: The trinitrobenzene sulfonate (TNBS) model of induced colitis was used in Balb/c mice. Subsequently after intravenous adeno-associated virus-mediated regulatory T-cell epitopes (Tregitope) delivery, acute colitis was initiated by intra-rectal administration of 1.5 mg TNBS in 40% ethanol followed by a second treatment with TNBS (0.75 mg in 20% ethanol) 8 d later. Control groups included mice not treated with TNBS (healthy control group) and mice treated by TNBS only (diseased group). At the time of sacrifice colon weight, the disease activity index and histology damage score were determined. Immunohistochemical staining of the colonic tissues was performed to asses the cellular infiltrate and the presence of transcription factor forkhead Box-P3 (Foxp3). Thymus, mesenteric lymph nodes, liver and spleen tissue were collected and the corresponding lymphocyte populations were further assessed by flow cytometry analysis for the expression of CD4+ T cell and regulatory T cell associated markers.
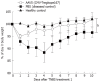
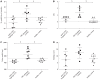
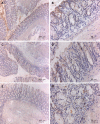
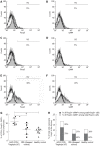
Results: The Tregitope 167 treated mice gained an average of 4% over their initial body weight at the time of sacrifice. In contrast, the mice treated with TNBS alone (no Tregitope) developed colitis, and lost 4% of their initial body weight at the time of sacrifice (P < 0.01). The body weight increase that had been observed in the mice pre-treated with Tregitope 167 was substantiated by a lower disease activity index and a decreased colon weight as compared to the diseased control group (P < 0.01 and P < 0.001, respectively). Immunohistochemical staining of the colonic tissues for CD4+ showed that inflammatory cell infiltrates were present in TNBS treated mice with or without administration with tregitope 167 and that these cellular infiltrates consisted mainly of CD4+ cells. For both TNBS treated groups CD4+ T cell infiltrates were observed in the sub-epithelial layer and the lamina propria. CD4+ T cell infiltrates were also present in the muscularis mucosa layer of the diseased control mice, but were absent in the Tregitope 167 treated group. Numerous Foxp3 positive cells were detected in the lamina propria and sub-epithelium of the colon sections from mice treated with Tregitope 167. Furthermore, the Foxp3 and glycoprotein A repetitions predominant markers were significantly increased in the CD4+ T lymphocyte population in the thymus of the mice pre-treated with adeno-associated virus serotype 5 (cytomegalovirus promoter-Tregitope 167), as cytomegalovirus promoter compared to lymphocyte populations in the thymus of diseased and the healthy control mice (P < 0.05 and P < 0.001, respectively).
Conclusion: This study identifies adeno-associated virus-mediated delivery of regulatory T-cell epitope 167 as a novel anti-inflammatory approach with the capacity to decrease intestinal inflammation and induce long-term remission in inflammatory bowel disease.
Keywords: Adeno-associated virus; Inflammatory bowel diseases; Regulatory T cell epitope.
Figures



Similar articles
-
Probiotics increase T regulatory cells and reduce severity of experimental colitis in mice.World J Gastroenterol. 2013 Feb 7;19(5):742-9. doi: 10.3748/wjg.v19.i5.742. World J Gastroenterol. 2013. PMID: 23430765 Free PMC article.
-
Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses.World J Gastroenterol. 2013 Aug 7;19(29):4702-17. doi: 10.3748/wjg.v19.i29.4702. World J Gastroenterol. 2013. PMID: 23922467 Free PMC article.
-
Inhibitor of PI3Kγ ameliorates TNBS-induced colitis in mice by affecting the functional activity of CD4+CD25+FoxP3+ regulatory T cells.Br J Pharmacol. 2011 May;163(2):358-74. doi: 10.1111/j.1476-5381.2011.01226.x. Br J Pharmacol. 2011. PMID: 21244371 Free PMC article.
-
Recovery from TNBS-induced colitis leads to the resistance to recurrent colitis and an increased ratio of FOXP3 to CD3 mRNA.J Dig Dis. 2013 Nov;14(11):587-95. doi: 10.1111/1751-2980.12084. J Dig Dis. 2013. PMID: 23786412
-
Effect of Trichinella spiralis intervention on TNBS-induced experimental colitis in mice.Immunobiology. 2019 Jan;224(1):147-153. doi: 10.1016/j.imbio.2018.09.005. Epub 2018 Oct 25. Immunobiology. 2019. PMID: 30413272
Cited by
-
Tregitopes Improve Asthma by Promoting Highly Suppressive and Antigen-Specific Tregs.Front Immunol. 2021 Apr 19;12:634509. doi: 10.3389/fimmu.2021.634509. eCollection 2021. Front Immunol. 2021. PMID: 33953711 Free PMC article.
-
Tregitope peptides: the active pharmaceutical ingredient of IVIG?Clin Dev Immunol. 2013;2013:493138. doi: 10.1155/2013/493138. Epub 2013 Dec 25. Clin Dev Immunol. 2013. PMID: 24454476 Free PMC article. Review.
-
A SARS-CoV-2 NSP7 homolog of a Treg epitope suppresses CD4+ and CD8+ T cell memory responses.Front Immunol. 2023 Nov 24;14:1290688. doi: 10.3389/fimmu.2023.1290688. eCollection 2023. Front Immunol. 2023. PMID: 38124752 Free PMC article.
-
Cell-Mediated Immunity to AAV Vectors, Evolving Concepts and Potential Solutions.Front Immunol. 2014 Jul 23;5:350. doi: 10.3389/fimmu.2014.00350. eCollection 2014. Front Immunol. 2014. PMID: 25101090 Free PMC article. Review.
-
The curious case of AAV immunology.Mol Ther. 2025 May 7;33(5):1946-1965. doi: 10.1016/j.ymthe.2025.03.037. Epub 2025 Mar 27. Mol Ther. 2025. PMID: 40156190 Review.
References
-
- Lakatos L, Kiss LS, David G, Pandur T, Erdelyi Z, Mester G, Balogh M, Szipocs I, Molnar C, Komaromi E, et al. Incidence, disease phenotype at diagnosis, and early disease course in inflammatory bowel diseases in Western Hungary, 2002-2006. Inflamm Bowel Dis. 2011;17:2558–2565. - PubMed
-
- Loftus EV, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1–20. - PubMed
-
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

