FOLFIRI regimen in metastatic pancreatic adenocarcinoma resistant to gemcitabine and platinum-salts
- PMID: 22969226
- PMCID: PMC3435778
- DOI: 10.3748/wjg.v18.i33.4533
FOLFIRI regimen in metastatic pancreatic adenocarcinoma resistant to gemcitabine and platinum-salts
Abstract
Aim: To evaluate the efficacy and safety of the FOLFIRI regimen in patients with metastatic pancreatic adenocarcinoma (PAC) after the failure of gemcitabine and platinum salts.
Methods: All consecutive patients with histologically confirmed, metastatic PAC and World Health Organization performance status (PS) ≤ 2 received FOLFIRI-1 [irinotecan 180 mg/m(2) on day 1 and leucovorin 400 mg/m(2) followed by 5-fluorouracil (5-FU) 400 mg/m(2) bolus, then 5-FU 2400 mg/m(2) as a 46-h infusion, biweekly] or FOLFIRI-3 (irinotecan 100 mg/m(2) on day 1 and leucovorin 400 mg/m(2), then 5-FU 2400 mg/m(2) as a 46-h infusion and irinotecan 100 mg/m(2) repeated on day 3, biweekly) after failure of gemcitabine and platinum-based chemotherapies as a systematic policy in two institutions between January 2005 and May 2010. Tumor response, time to progression (TTP), overall survival rate (OS) and grade 3-4 toxicities were retrospectively studied. Subgroup analyses were performed to search for prognostic factors.
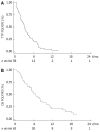
Results: Sixty-three patients (52.4% male, median age 59 years) were analyzed. Among them, 42.9% were PS 0, 38.1% were PS 1 and 19.0% were PS 2. Fifty one patients (81.0%) had liver metastases. Before the FOLFIRI regimen, patients had received 1 line (n = 19), 2 lines (n = 39) or 3 lines (n = 5) of chemotherapy. Median TTP obtained with the line before FOLFIRI was 3.9 mo (95% CI: 3.4-5.3 mo). A total of 480 cycles was completed (median: 6 cycles, range: 1-51 cycles). The main reason for discontinuing FOLFIRI was tumor progression (90.3%). Tumor control was achieved in 25 patients (39.7%) (partial response: n = 5, stable disease: n = 20) with FOLFIRI. Median TTP was 3.0 mo (95% CI: 2.1-3.9 mo) and median OS was 6.6 mo (95% CI: 5.3-8.1 mo). Dose adaptation was required in 36 patients (57.1%). Fifteen patients (23.8%) had grade 3-4 toxicities, mainly hematological (n = 11) or digestive (n = 4). Febrile neutropenia occurred in 3 patients. There was no toxic death. PS 2 was significantly associated with poor TTP [hazard ratio (HR): 16.036, P < 0.0001] and OS (HR: 4.003, P = 0.004).
Conclusion: The FOLFIRI regimen had an acceptable toxicity and an interesting efficacy in our study, limited to patients in good condition (PS 0-1).
Keywords: 5-fluorouracil; Camptothecin; Chemotherapy; FOLFIRI regimen; Irinotecan; Metastases; Pancreatic adenocarcinoma; Pancreatic cancer.
Figures

References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. - PubMed
-
- Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009;45:931–991. - PubMed
-
- Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Di Marco M, Di Cicilia R, Macchini M, Nobili E, Vecchiarelli S, Brandi G, Biasco G. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? (Review) Oncol Rep. 2010;23:1183–1192. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

