7-difluoromethoxyl-5,4'-di-n-octylgenistein inhibits growth of gastric cancer cells through downregulating forkhead box M1
- PMID: 22969238
- PMCID: PMC3435790
- DOI: 10.3748/wjg.v18.i33.4618
7-difluoromethoxyl-5,4'-di-n-octylgenistein inhibits growth of gastric cancer cells through downregulating forkhead box M1
Abstract
Aim: To investigate whether the 7-difluoromethoxyl-5, 4'-di-n-octylgenistein (DFOG), a novel synthetic genistein analogue, affects the growth of gastric cancer cells and its mechanisms.
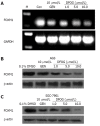
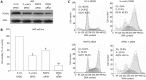
Methods: A series of genistein analogues were prepared by difluoromethylation and alkylation, and human gastric cancer cell lines AGS and SGC-7901 cultured in vitro were treated with various concentrations of genistein and genistein analogues. The cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The cells were incubated by DFOG at different concentrations. The growth inhibitory effects were evaluated using MTT and clonogenic assay. The distribution of the phase in cell cycle was analyzed using flow cytometric analysis with propidium iodide staining. The expression of the transcription factor forkhead box M1 (FOXM1) was analyzed by reverse transcription-polymerase chain reaction and Western blotting. The expression levels of CDK1, Cdc25B, cyclin B and p27(KIP1) protein were detected using Western blotting.
Results: Nine of the genistein analogues had more effective antitumor activity than genistein. Among the tested analogues, DFOG possessed the strongest activity against AGS and SGC-7901 cells in vitro. DFOG significantly inhibited the cell viability and colony formation of AGS and SGC-7901 cells. Moreover, DFOG efficaciously arrested the cell cycle in G2/M phase. DFOG decreased the expression of FOXM1 and its downstream genes, such as CDK1, Cdc25B, cyclin B, and increased p27(KIP1) at protein levels. Knockdown of FOXM1 by small interfering RNA before DFOG treatment resulted in enhanced cell growth inhibition in AGS cells. Up-regulation of FOXM1 by cDNA transfection attenuated DFOG-induced cell growth inhibition in AGS cells.
Conclusion: DFOG inhibits the growth of human gastric cancer cells by down-regulating the FOXM1 expression.
Keywords: 7-difluoromethoxyl-5,4’-di-n-octylgenistein; Forkhead box M1; Gastric cancer; Genistein; Therapeutic action.
Figures





Similar articles
-
Apoptosis induced by 7-difluoromethoxyl-5,4'-di-n-octyl genistein via the inactivation of FoxM1 in ovarian cancer cells.Oncol Rep. 2012 Jun;27(6):1857-64. doi: 10.3892/or.2012.1739. Epub 2012 Mar 22. Oncol Rep. 2012. PMID: 22447287
-
7-Difluoromethoxyl-5,4'-di-n-octyl genistein inhibits the stem-like characteristics of gastric cancer stem-like cells and reverses the phenotype of epithelial-mesenchymal transition in gastric cancer cells.Oncol Rep. 2016 Aug;36(2):1157-65. doi: 10.3892/or.2016.4848. Epub 2016 Jun 2. Oncol Rep. 2016. PMID: 27279287
-
Disruption of crosstalk between LX-2 and liver cancer stem-like cells from MHCC97H cells by DFOG via inhibiting FOXM1.Acta Biochim Biophys Sin (Shanghai). 2019 Dec 13;51(12):1267-1275. doi: 10.1093/abbs/gmz129. Acta Biochim Biophys Sin (Shanghai). 2019. PMID: 31750892
-
FOX(M1) news--it is cancer.Mol Cancer Ther. 2013 Mar;12(3):245-54. doi: 10.1158/1535-7163.MCT-12-0712. Epub 2013 Feb 26. Mol Cancer Ther. 2013. PMID: 23443798 Free PMC article. Review.
-
The role of FoxM1 in immune cells.Clin Exp Med. 2023 Oct;23(6):1973-1979. doi: 10.1007/s10238-023-01037-w. Epub 2023 Mar 13. Clin Exp Med. 2023. PMID: 36913035 Review.
Cited by
-
Therapeutic vulnerabilities of cancer stem cells and effects of natural products.Nat Prod Rep. 2023 Aug 16;40(8):1432-1456. doi: 10.1039/d3np00002h. Nat Prod Rep. 2023. PMID: 37103550 Free PMC article. Review.
-
Genistein inhibits lung cancer cell stem-like characteristics by modulating MnSOD and FoxM1 expression.Oncol Lett. 2020 Sep;20(3):2506-2515. doi: 10.3892/ol.2020.11802. Epub 2020 Jul 1. Oncol Lett. 2020. Retraction in: Oncol Lett. 2025 May 19;30(1):351. doi: 10.3892/ol.2025.15097. PMID: 32782570 Free PMC article. Retracted.
-
Clinical significance and biological roles of cyclins in gastric cancer.Onco Targets Ther. 2018 Oct 9;11:6673-6685. doi: 10.2147/OTT.S171716. eCollection 2018. Onco Targets Ther. 2018. PMID: 30349301 Free PMC article.
-
7‑Difluoromethoxyl‑5,4'‑di‑n‑octylygenistein targets the STAT3 pathway by upregulating microRNA‑152‑3p expression to inhibit self‑renewal and tumor growth in non‑small cell lung carcinoma.Oncol Rep. 2025 Jun;53(6):66. doi: 10.3892/or.2025.8899. Epub 2025 Apr 17. Oncol Rep. 2025. PMID: 40242966 Free PMC article.
-
7-difluoromethoxyl-5,4'-di-n-octyl genistein inhibits ovarian cancer stem cell characteristics through the downregulation of FOXM1.Oncol Lett. 2014 Jul;8(1):295-300. doi: 10.3892/ol.2014.2080. Epub 2014 Apr 22. Oncol Lett. 2014. PMID: 24959264 Free PMC article.
References
-
- Refsnes M, Thrane EV, Låg M, Thoresen GH, Schwarze PE. Mechanisms in fluoride-induced interleukin-8 synthesis in human lung epithelial cells. Toxicology. 2001;167:145–158. - PubMed
-
- Zheng X, Meng WD, Xu YY, Cao JG, Qing FL. Synthesis and anticancer effect of chrysin derivatives. Bioorg Med Chem Lett. 2003;13:881–884. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

