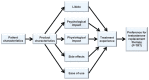
Development of a men's Preference for Testosterone Replacement Therapy (P-TRT) instrument
- PMID: 22969294
- PMCID: PMC3437909
- DOI: 10.2147/PPA.S35840
Development of a men's Preference for Testosterone Replacement Therapy (P-TRT) instrument
Abstract
Background: This study used a standard research approach to create a final conceptual model and the Preference for the Testosterone Replacement Therapy (P-TRT) instrument.
Methods: A discussion guide was developed from a literature review and expert opinion to direct one-on-one interviews with participants who used testosterone replacement therapy and consented to participate in the study. Data from telephone interviews were transcribed for theme analysis using NVivo 9 qualitative analysis software, analyzed descriptively from a saturation grid, and used to evaluate men's P-TRT. Data from cognitive debriefing for five participants were used to evaluate the final conceptual model and validate the initial P-TRT instrument.
Results: Item saturation and theme exhaustion was achieved by 58 male participants of mean age 55.0 ± 10.0 (22-69) years who had used testosterone replacement therapy for a mean of 175.0 ± 299.2 days. The conceptual model was developed from items and themes obtained from the participant interviews and saturation grid. Items comprising eight dimensions were used for instrument development, ie, ease of use, effect on libido, product characteristics, physiological impact, psychological impact, side effects, treatment experience, and preference. Results from the testosterone replacement therapy preference evaluation provide a detailed insight into why most men preferred a topical gel product over an injection or patch.
Conclusion: Items and themes relating to use of testosterone replacement therapy were in concordance with the final conceptual model and 29-item P-TRT instrument. The standard research approach used in this study produced the P-TRT instrument, which is suitable for further psychometric development and use in clinical practice.
Keywords: hormones; hypogonadism; outcome assessment; patient preference; testosterone.
Figures
Similar articles
-
Failure of testosterone replacement therapy to improve symptoms correlates with burden of systemic conditions.Transl Androl Urol. 2020 Jun;9(3):1108-1112. doi: 10.21037/tau-19-848. Transl Androl Urol. 2020. PMID: 32676394 Free PMC article.
-
EMAS position statement: Testosterone replacement therapy in older men.Maturitas. 2023 Dec;178:107854. doi: 10.1016/j.maturitas.2023.107854. Epub 2023 Oct 15. Maturitas. 2023. PMID: 37845136
-
Testosterone and aging male, a perspective from a developing country.Aging Male. 2023 Dec;26(1):2223712. doi: 10.1080/13685538.2023.2223712. Aging Male. 2023. PMID: 37335039 Review.
-
The effect of physiotherapy in addition to testosterone replacement therapy on the efficiency of the motor system in men with hypogonadism.Medicina (Kaunas). 2013;49(2):71-7. Medicina (Kaunas). 2013. PMID: 23888342 Clinical Trial.
-
Testosterone replacement therapy.Andrology. 2020 Nov;8(6):1551-1566. doi: 10.1111/andr.12774. Epub 2020 Mar 9. Andrology. 2020. PMID: 32068334 Review.
Cited by
-
The Pre-Testosterone Therapy Checklist.J Sex Med. 2022 Aug;19(8):1214-1217. doi: 10.1016/j.jsxm.2022.03.619. Epub 2022 May 2. J Sex Med. 2022. PMID: 35514000 Free PMC article.
-
The impact of drug reimbursement policy on rates of testosterone replacement therapy among older men.PLoS One. 2014 Jul 16;9(7):e98003. doi: 10.1371/journal.pone.0098003. eCollection 2014. PLoS One. 2014. PMID: 25029014 Free PMC article.
-
Physicians' attitudes towards androgen replacement therapy for male and female sexual dysfunction.Int J Impot Res. 2016 Mar-Apr;28(2):57-60; quiz 60-1. doi: 10.1038/ijir.2016.2. Epub 2016 Feb 11. Int J Impot Res. 2016. PMID: 26865099
-
Identifying the outcomes important to men with hypogonadism: A qualitative evidence synthesis.Andrology. 2022 May;10(4):625-641. doi: 10.1111/andr.13156. Epub 2022 Feb 8. Andrology. 2022. PMID: 35064779 Free PMC article. Review.
-
Transdermal testosterone replacement therapy in men.Drug Des Devel Ther. 2014 Jan 9;8:101-12. doi: 10.2147/DDDT.S43475. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 24470750 Free PMC article. Review.
References
-
- Araujo AB, O’Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts male aging study. J Clin Endocrinol Metab. 2004;89(12):5920–5926. - PubMed
-
- Wu FC, Tajar A, Beynon JM, et al. EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–135. - PubMed
-
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241–4247. - PubMed
-
- Bhasin S, Basaria S. Diagnosis and treatment of hypogonadism is men. Best Pract Res Clin Endocrinol Metab. 2011;25(2):251–270. - PubMed