Btg1 is Required to Maintain the Pool of Stem and Progenitor Cells of the Dentate Gyrus and Subventricular Zone
- PMID: 22969701
- PMCID: PMC3431174
- DOI: 10.3389/fnins.2012.00124
Btg1 is Required to Maintain the Pool of Stem and Progenitor Cells of the Dentate Gyrus and Subventricular Zone
Abstract
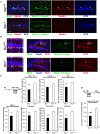
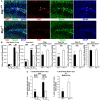
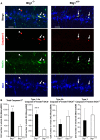
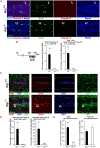
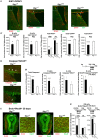
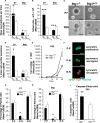
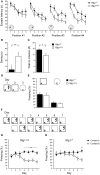
Btg1 belongs to a family of cell cycle inhibitory genes. We observed that Btg1 is highly expressed in adult neurogenic niches, i.e., the dentate gyrus and subventricular zone (SVZ). Thus, we generated Btg1 knockout mice to analyze the role of Btg1 in the process of generation of adult new neurons. Ablation of Btg1 causes a transient increase of the proliferating dentate gyrus stem and progenitor cells at post-natal day 7; however, at 2 months of age the number of these proliferating cells, as well as of mature neurons, greatly decreases compared to wild-type controls. Remarkably, adult dentate gyrus stem and progenitor cells of Btg1-null mice exit the cell cycle after completing the S phase, express p53 and p21 at high levels and undergo apoptosis within 5 days. In the SVZ of adult (two-month-old) Btg1-null mice we observed an equivalent decrease, associated to apoptosis, of stem cells, neuroblasts, and neurons; furthermore, neurospheres derived from SVZ stem cells showed an age-dependent decrease of the self-renewal and expansion capacity. We conclude that ablation of Btg1 reduces the pool of dividing adult stem and progenitor cells in the dentate gyrus and SVZ by decreasing their proliferative capacity and inducing apoptosis, probably reflecting impairment of the control of the cell cycle transition from G1 to S phase. As a result, the ability of Btg1-null mice to discriminate among overlapping contextual memories was affected. Btg1 appears, therefore, to be required for maintaining adult stem and progenitor cells quiescence and self-renewal.
Keywords: BTG family; differentiation; knock out mice; learning and memory; neural stem cells; neurogenic niches; proliferation.
Figures


References
-
- Arguello A. A., Harburg G. C., Schonborn J. R., Mandyam C. D., Yamaguchi M., Eisch A. J. (2008). Time course of morphine’s effects on adult hippocampal subgranular zone reveals preferential inhibition of cells in S phase of the cell cycle and a subpopulation of immature neurons. Neuroscience 157, 70–79 10.1016/j.neuroscience.2008.08.064 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

