Single-neuron diversity generated by Protocadherin-β cluster in mouse central and peripheral nervous systems
- PMID: 22969705
- PMCID: PMC3431597
- DOI: 10.3389/fnmol.2012.00090
Single-neuron diversity generated by Protocadherin-β cluster in mouse central and peripheral nervous systems
Abstract
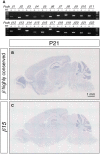
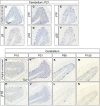
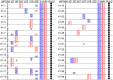
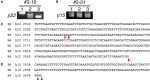
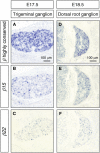
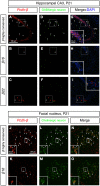
The generation of complex neural circuits depends on the correct wiring of neurons with diverse individual characteristics. To understand the complexity of the nervous system, the molecular mechanisms for specifying the identity and diversity of individual neurons must be elucidated. The clustered protocadherins (Pcdh) in mammals consist of approximately 50 Pcdh genes (Pcdh-α, Pcdh-β, and Pcdh-γ) that encode cadherin-family cell surface adhesion proteins. Individual neurons express a random combination of Pcdh-α and Pcdh-γ, whereas the expression patterns for the Pcdh-β genes, 22 one-exon genes in mouse, are not fully understood. Here we show that the Pcdh-β genes are expressed in a 3'-polyadenylated form in mouse brain. In situ hybridization using a pan-Pcdh-β probe against a conserved Pcdh-β sequence showed widespread labeling in the brain, with prominent signals in the olfactory bulb, hippocampus, and cerebellum. In situ hybridization with specific probes for individual Pcdh-β genes showed their expression to be scattered in Purkinje cells from P10 to P150. The scattered expression patterns were confirmed by performing a newly developed single-cell 3'-RACE analysis of Purkinje cells, which clearly demonstrated that the Pcdh-β genes are expressed monoallelically and combinatorially in individual Purkinje cells. Scattered expression patterns of individual Pcdh-β genes were also observed in pyramidal neurons in the hippocampus and cerebral cortex, neurons in the trigeminal and dorsal root ganglion, GABAergic interneurons, and cholinergic neurons. Our results extend previous observations of diversity at the single-neuron level generated by Pcdh expression and suggest that the Pcdh-β cluster genes contribute to specifying the identity and diversity of individual neurons.
Keywords: Pcdh; combinatorial expression; monoallelic; neural circuit; neuronal individuality; protocadherin; single-cell 3′-RACE; single-neuron diversity.
Figures


Similar articles
-
Clustered protocadherin family.Dev Growth Differ. 2008 Jun;50 Suppl 1:S131-40. doi: 10.1111/j.1440-169X.2008.00991.x. Epub 2008 Apr 22. Dev Growth Differ. 2008. PMID: 18430161 Review.
-
Combinatorial Effects of Alpha- and Gamma-Protocadherins on Neuronal Survival and Dendritic Self-Avoidance.J Neurosci. 2018 Mar 14;38(11):2713-2729. doi: 10.1523/JNEUROSCI.3035-17.2018. Epub 2018 Feb 8. J Neurosci. 2018. PMID: 29439167 Free PMC article.
-
Molecular codes for neuronal individuality and cell assembly in the brain.Front Mol Neurosci. 2012 Apr 12;5:45. doi: 10.3389/fnmol.2012.00045. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22518100 Free PMC article.
-
The role and expression of the protocadherin-alpha clusters in the CNS.Curr Opin Neurobiol. 2006 Jun;16(3):336-42. doi: 10.1016/j.conb.2006.05.003. Epub 2006 May 11. Curr Opin Neurobiol. 2006. PMID: 16697637 Review.
-
Identification of the cluster control region for the protocadherin-beta genes located beyond the protocadherin-gamma cluster.J Biol Chem. 2011 Sep 9;286(36):31885-95. doi: 10.1074/jbc.M111.245605. Epub 2011 Jul 19. J Biol Chem. 2011. PMID: 21771796 Free PMC article.
Cited by
-
γ-Protocadherin structural diversity and functional implications.Elife. 2016 Oct 26;5:e20930. doi: 10.7554/eLife.20930. Elife. 2016. PMID: 27782885 Free PMC article.
-
Clustered protocadherins.Development. 2013 Aug;140(16):3297-302. doi: 10.1242/dev.090621. Development. 2013. PMID: 23900538 Free PMC article. Review.
-
The role of clustered protocadherins in neurodevelopment and neuropsychiatric diseases.Curr Opin Genet Dev. 2020 Dec;65:144-150. doi: 10.1016/j.gde.2020.05.041. Epub 2020 Jul 14. Curr Opin Genet Dev. 2020. PMID: 32679536 Free PMC article. Review.
-
Development of FRET-based indicators for visualizing homophilic trans interaction of a clustered protocadherin.Sci Rep. 2021 Nov 15;11(1):22237. doi: 10.1038/s41598-021-01481-2. Sci Rep. 2021. PMID: 34782670 Free PMC article.
-
Structural origins of clustered protocadherin-mediated neuronal barcoding.Semin Cell Dev Biol. 2017 Sep;69:140-150. doi: 10.1016/j.semcdb.2017.07.023. Epub 2017 Jul 22. Semin Cell Dev Biol. 2017. PMID: 28743640 Free PMC article. Review.
References
-
- Arvidsson U., Riedl M., Elde R., Meister B. (1997). Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J. Comp. Neurol. 378, 454–467 10.1002/(SICI)1096-9861(19970224)378:4<454::AID-CNE2>3.0.CO;2-1 - DOI - PubMed
-
- Castillo S. D., Matheu A., Mariani N., Carretero J., Lopez-Rios F., Lovell-Badge R., Sanchez-Cespedes M. (2012). Novel transcriptional targets of the SRY-HMG box transcription factor SOX4 link its expression to the development of small cell lung cancer. Cancer Res. 72, 176–186 10.1158/0008-5472.CAN-11-3506 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

