Direction selectivity in the larval zebrafish tectum is mediated by asymmetric inhibition
- PMID: 22969706
- PMCID: PMC3432856
- DOI: 10.3389/fncir.2012.00059
Direction selectivity in the larval zebrafish tectum is mediated by asymmetric inhibition
Abstract
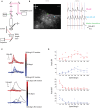
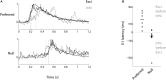
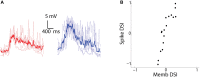
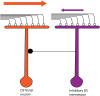
The extraction of the direction of motion is an important computation performed by many sensory systems and in particular, the mechanism by which direction-selective retinal ganglion cells (DS-RGCs) in the retina acquire their selective properties, has been studied extensively. However, whether DS-RGCs simply relay this information to downstream areas or whether additional and potentially de novo processing occurs in these recipient structures is a matter of great interest. Neurons in the larval zebrafish tectum, the largest retino-recipent area in this animal, show direction-selective (DS) responses to moving visual stimuli but how these properties are acquired is still unknown. In order to study this, we first used two-photon calcium imaging to classify the population responses of tectal cells to bars moving at different speeds and in different directions. Subsequently, we performed in vivo whole cell electrophysiology on these DS tectal neurons and we found that their inhibitory inputs were strongly biased toward the null direction of motion, whereas the excitatory inputs showed little selectivity. In addition, we found that excitatory currents evoked by a stimulus moving in the preferred direction occurred before the inhibitory currents whereas a stimulus moving in the null direction evoked currents in the reverse temporal order. The membrane potential modulations resulting from these currents were enhanced by the spike generation mechanism to generate amplified direction selectivity in the spike output. Thus, our results implicate a local inhibitory circuit in generating direction selectivity in tectal neurons.
Keywords: asymmetric inhibition; direction selectivity; tectum; vision; zebrafish.
Figures


Similar articles
-
A Three-Layer Network Model of Direction Selective Circuits in the Optic Tectum.Front Neural Circuits. 2017 Nov 21;11:88. doi: 10.3389/fncir.2017.00088. eCollection 2017. Front Neural Circuits. 2017. PMID: 29209178 Free PMC article.
-
Optic tectal superficial interneurons detect motion in larval zebrafish.Protein Cell. 2019 Apr;10(4):238-248. doi: 10.1007/s13238-018-0587-7. Epub 2018 Nov 12. Protein Cell. 2019. PMID: 30421356 Free PMC article.
-
Direction selectivity in the visual system of the zebrafish larva.Front Neural Circuits. 2013 Jun 18;7:111. doi: 10.3389/fncir.2013.00111. eCollection 2013. Front Neural Circuits. 2013. PMID: 23785314 Free PMC article. Review.
-
Layer-specific targeting of direction-selective neurons in the zebrafish optic tectum.Neuron. 2012 Dec 20;76(6):1147-60. doi: 10.1016/j.neuron.2012.12.003. Neuron. 2012. PMID: 23259950
-
Mathematical analysis and modeling of motion direction selectivity in the retina.J Physiol Paris. 2013 Nov;107(5):349-59. doi: 10.1016/j.jphysparis.2013.08.003. Epub 2013 Sep 2. J Physiol Paris. 2013. PMID: 24008129 Review.
Cited by
-
Synaptic Effects of Dopamine Breakdown and Their Relation to Schizophrenia-Linked Working Memory Deficits.Front Synaptic Neurosci. 2018 Jun 12;10:16. doi: 10.3389/fnsyn.2018.00016. eCollection 2018. Front Synaptic Neurosci. 2018. PMID: 29950984 Free PMC article.
-
Emergent properties of the optic tectum revealed by population analysis of direction and orientation selectivity.J Neurosci. 2013 Aug 28;33(35):13940-5. doi: 10.1523/JNEUROSCI.1493-13.2013. J Neurosci. 2013. PMID: 23986231 Free PMC article.
-
Updated functional segregation of retinal ganglion cell projections in the tectum of a cyprinid fish-further elaboration based on microelectrode recordings.Fish Physiol Biochem. 2019 Apr;45(2):773-792. doi: 10.1007/s10695-018-0603-0. Epub 2019 Jan 5. Fish Physiol Biochem. 2019. PMID: 30612338
-
A Three-Layer Network Model of Direction Selective Circuits in the Optic Tectum.Front Neural Circuits. 2017 Nov 21;11:88. doi: 10.3389/fncir.2017.00088. eCollection 2017. Front Neural Circuits. 2017. PMID: 29209178 Free PMC article.
-
Retinal ganglion cell maps in the brain: implications for visual processing.Curr Opin Neurobiol. 2014 Feb;24(1):133-42. doi: 10.1016/j.conb.2013.08.006. Epub 2013 Nov 19. Curr Opin Neurobiol. 2014. PMID: 24492089 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

