Error awareness and salience processing in the oddball task: shared neural mechanisms
- PMID: 22969714
- PMCID: PMC3427876
- DOI: 10.3389/fnhum.2012.00246
Error awareness and salience processing in the oddball task: shared neural mechanisms
Abstract
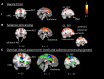
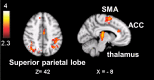
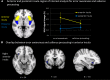
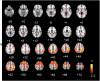
A body of work suggests similarities in the way we become aware of an error and process motivationally salient events. Yet, evidence for a shared neural mechanism has not been provided. A within subject investigation of the brain regions involved in error awareness and salience processing has not been reported. While the neural response to motivationally salient events is classically studied during target detection after longer target-to-target intervals in an oddball task and engages a widespread insula-thalamo-cortical brain network, error awareness has recently been linked to, most prominently, anterior insula cortex. Here we explore whether the anterior insula activation for error awareness is related to salience processing, by testing for activation overlap in subjects undergoing two different task settings. Using a within subjects design, we show activation overlap in six major brain areas during aware errors in an antisaccade task and during target detection after longer target-to-target intervals in an oddball task: anterior insula, anterior cingulate, supplementary motor area, thalamus, brainstem, and parietal lobe. Within subject analyses shows that the insula is engaged in both error awareness and the processing of salience, and that the anterior insula is more involved in both processes than the posterior insula. The results of a fine-grained spatial pattern overlap analysis between active clusters in the same subjects indicates that even if the anterior insula is activated for both error awareness and salience processing, the two types of processes might tend to activate non-identical neural ensembles on a finer-grained spatial level. Together, these outcomes suggest a similar functional phenomenon in the two different task settings. Error awareness and salience processing share a functional anatomy, with a tendency toward subregional dorsal and ventral specialization within the anterior insula.
Keywords: anterior insula; error awareness; eyetracking; magnetic resonance imaging; oddball processing; salience.
Figures


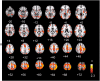
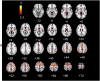
Similar articles
-
Salience network connectivity in the insula is associated with individual differences in interoceptive accuracy.Brain Struct Funct. 2017 May;222(4):1635-1644. doi: 10.1007/s00429-016-1297-7. Epub 2016 Aug 29. Brain Struct Funct. 2017. PMID: 27573028
-
Error blindness and motivational significance: Shifts in networks centering on anterior insula co-vary with error awareness and pupil dilation.Behav Brain Res. 2018 Dec 14;355:24-35. doi: 10.1016/j.bbr.2017.10.030. Epub 2017 Oct 26. Behav Brain Res. 2018. PMID: 29107022
-
Examining the neural correlates of error awareness in a large fMRI study.Cereb Cortex. 2022 Dec 20;33(2):458-468. doi: 10.1093/cercor/bhac077. Cereb Cortex. 2022. PMID: 35238340 Free PMC article.
-
Saliency, switching, attention and control: a network model of insula function.Brain Struct Funct. 2010 Jun;214(5-6):655-67. doi: 10.1007/s00429-010-0262-0. Epub 2010 May 29. Brain Struct Funct. 2010. PMID: 20512370 Free PMC article. Review.
-
Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task.Cereb Cortex. 2004 Sep;14(9):986-94. doi: 10.1093/cercor/bhh059. Epub 2004 Apr 27. Cereb Cortex. 2004. PMID: 15115734 Review.
Cited by
-
Editorial for E-Book: error awareness-insights from cognitive neuroscience, psychiatry and neurology.Front Hum Neurosci. 2013 Dec 5;7:830. doi: 10.3389/fnhum.2013.00830. eCollection 2013. Front Hum Neurosci. 2013. PMID: 24367313 Free PMC article. No abstract available.
-
Spatiotemporal neural dynamics of moral judgment: a high-density ERP study.Neuropsychologia. 2014 Jul;60:39-45. doi: 10.1016/j.neuropsychologia.2014.05.022. Epub 2014 Jun 4. Neuropsychologia. 2014. PMID: 24905282 Free PMC article.
-
Motivational Deficits in Schizophrenia and the Representation of Expected Value.Curr Top Behav Neurosci. 2016;27:375-410. doi: 10.1007/7854_2015_385. Curr Top Behav Neurosci. 2016. PMID: 26370946 Free PMC article. Review.
-
The Good, the bad, and the just: justice sensitivity predicts neural response during moral evaluation of actions performed by others.J Neurosci. 2014 Mar 19;34(12):4161-6. doi: 10.1523/JNEUROSCI.4648-13.2014. J Neurosci. 2014. PMID: 24647937 Free PMC article.
-
Neuromodulation of OCD: A review of invasive and non-invasive methods.Front Neurol. 2022 Aug 9;13:909264. doi: 10.3389/fneur.2022.909264. eCollection 2022. Front Neurol. 2022. PMID: 36016538 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

