Fusimotor control of spindle sensitivity regulates central and peripheral coding of joint angles
- PMID: 22969720
- PMCID: PMC3431011
- DOI: 10.3389/fncom.2012.00066
Fusimotor control of spindle sensitivity regulates central and peripheral coding of joint angles
Abstract
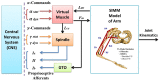
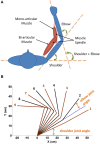
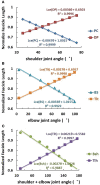
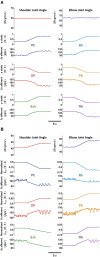
Proprioceptive afferents from muscle spindles encode information about peripheral joint movements for the central nervous system (CNS). The sensitivity of muscle spindle is nonlinearly dependent on the activation of gamma (γ) motoneurons in the spinal cord that receives inputs from the motor cortex. How fusimotor control of spindle sensitivity affects proprioceptive coding of joint position is not clear. Furthermore, what information is carried in the fusimotor signal from the motor cortex to the muscle spindle is largely unknown. In this study, we addressed the issue of communication between the central and peripheral sensorimotor systems using a computational approach based on the virtual arm (VA) model. In simulation experiments within the operational range of joint movements, the gamma static commands (γ(s)) to the spindles of both mono-articular and bi-articular muscles were hypothesized (1) to remain constant, (2) to be modulated with joint angles linearly, and (3) to be modulated with joint angles nonlinearly. Simulation results revealed a nonlinear landscape of Ia afferent with respect to both γ(s) activation and joint angle. Among the three hypotheses, the constant and linear strategies did not yield Ia responses that matched the experimental data, and therefore, were rejected as plausible strategies of spindle sensitivity control. However, if γ(s) commands were quadratically modulated with joint angles, a robust linear relation between Ia afferents and joint angles could be obtained in both mono-articular and bi-articular muscles. With the quadratic strategy of spindle sensitivity control, γ(s) commands may serve as the CNS outputs that inform the periphery of central coding of joint angles. The results suggest that the information of joint angles may be communicated between the CNS and muscles via the descending γ(s) efferent and Ia afferent signals.
Keywords: Ia afferents; central and peripheral coding; joint angle; muscle spindle; spindle sensitivity; γs control.
Figures
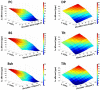

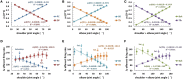
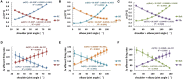
References
-
- Boyd I. A. (1980). The isolated mammalian muscle spindle. TINS 258–265
-
- Boyd I. A., Murphy P. R., Moss V. A. (1985). Analysis of primary and secondary afferent responses to stretch during activation of the dynamic bag1 fiber or the static bag2 fiber in cat muscle spindle, in The Muscle Spindle, eds Boyd I. A., Gladden M. H. (London: Macmillan; ), 153–158
-
- Boyd I. A., Smith R. S. (1984). The muscle spindle, in Peripheral Neuropathy, 2nd Edn, eds Dyck R. J., Thomas P. K., Lombert E. H., Bunge R. (Philadelphia, PA: W.B. Saunders Company; ), 171–202
-
- Corcos D. M., Gottlieb G. L., Agarwal G. C. (1989). Organizing principles for single joint movements, II. A speed-sensitive strategy. J. Neurophysiol. 62, 358–368 - PubMed
LinkOut - more resources
Full Text Sources

