A tale with a Twist: a developmental gene with potential relevance for metabolic dysfunction and inflammation in adipose tissue
- PMID: 22969750
- PMCID: PMC3430876
- DOI: 10.3389/fendo.2012.00108
A tale with a Twist: a developmental gene with potential relevance for metabolic dysfunction and inflammation in adipose tissue
Abstract
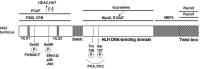
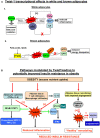
The Twist proteins (Twist-1 and -2) are highly conserved developmental proteins with key roles for the transcriptional regulation in mesenchymal cell lineages. They belong to the super-family of bHLH proteins and exhibit bi-functional roles as both activators and repressors of gene transcription. The Twist proteins are expressed at low levels in adult tissues but may become abundantly re-expressed in cells undergoing malignant transformation. This observation prompted extensive research on the roles of Twist proteins in cancer progression and metastasis. Very recent studies indicate a novel role for Twist-1 as a potential regulator of adipose tissue (AT) remodeling and inflammation. Several studies suggested that developmental genes are important determinants of obesity, fat distribution and remodeling capacity of different adipose depots. Twist-1 is abundantly and selectively expressed in the adult AT and its constitutive expression is significantly higher in subcutaneous (SAT) vs. visceral (VAT) fat in both mice and humans. Moreover, Twist1 expression is strongly correlated with BMI and insulin resistance in humans. However, the functional roles and transcriptional downstream targets of Twist1 in AT are largely unexplored. The purpose of this review is to highlight the major findings related to Twist1 expression in different fat depots and cellular components of AT and to discuss the potential mechanisms suggesting a role for Twist1 in AT metabolism, inflammation and remodeling.
Keywords: IL12; T cells; Twist1; human obesity; inflammation; type 2 diabetes; visceral fat.
Figures
Similar articles
-
Co-expressed immune and metabolic genes in visceral and subcutaneous adipose tissue from severely obese individuals are associated with plasma HDL and glucose levels: a microarray study.BMC Med Genomics. 2010 Aug 5;3:34. doi: 10.1186/1755-8794-3-34. BMC Med Genomics. 2010. PMID: 20687939 Free PMC article.
-
Two Faces of White Adipose Tissue with Heterogeneous Adipogenic Progenitors.Diabetes Metab J. 2019 Dec;43(6):752-762. doi: 10.4093/dmj.2019.0174. Diabetes Metab J. 2019. PMID: 31902145 Free PMC article. Review.
-
The human visceral fat depot has a unique inflammatory profile.Obesity (Silver Spring). 2010 May;18(5):879-83. doi: 10.1038/oby.2010.22. Obesity (Silver Spring). 2010. PMID: 20186138
-
Gene expression regional differences in human subcutaneous adipose tissue.BMC Genomics. 2017 Feb 23;18(1):202. doi: 10.1186/s12864-017-3564-2. BMC Genomics. 2017. PMID: 28231762 Free PMC article.
-
Redundant or separate entities?--roles of Twist1 and Twist2 as molecular switches during gene transcription.Nucleic Acids Res. 2011 Mar;39(4):1177-86. doi: 10.1093/nar/gkq890. Epub 2010 Oct 8. Nucleic Acids Res. 2011. PMID: 20935057 Free PMC article. Review.
Cited by
-
Roles of Twist1 in lipid and glucose metabolism.Cell Commun Signal. 2023 Oct 2;21(1):270. doi: 10.1186/s12964-023-01262-6. Cell Commun Signal. 2023. PMID: 37784111 Free PMC article. Review.
-
EMT Factors and Metabolic Pathways in Cancer.Front Oncol. 2020 Apr 7;10:499. doi: 10.3389/fonc.2020.00499. eCollection 2020. Front Oncol. 2020. PMID: 32318352 Free PMC article. Review.
-
Twist 1 regulates the expression of PPARγ during hormone-induced 3T3-L1 preadipocyte differentiation: a possible role in obesity and associated diseases.Lipids Health Dis. 2014 Aug 16;13:132. doi: 10.1186/1476-511X-13-132. Lipids Health Dis. 2014. PMID: 25128964 Free PMC article.
-
OVOL2: an epithelial lineage determiner with emerging roles in energy homeostasis.Trends Cell Biol. 2023 Oct;33(10):824-833. doi: 10.1016/j.tcb.2023.05.008. Epub 2023 Jun 17. Trends Cell Biol. 2023. PMID: 37336658 Free PMC article. Review.
-
The role of Twist1 in mutant huntingtin-induced transcriptional alterations and neurotoxicity.J Biol Chem. 2018 Jul 27;293(30):11850-11866. doi: 10.1074/jbc.RA117.001211. Epub 2018 Jun 11. J Biol Chem. 2018. PMID: 29891550 Free PMC article.
References
-
- Arner E., Mejhert N., Kulyte A., Balwierz P. J., Pachkov M., Cormont M., Lorente-Cebrian S., Ehrlund A., Laurencikiene J., Heden P., Dahlman-Wright K., Tanti J. F., Hayashizaki Y., Ryden M., Dahlman I., van Nimwegen E., Daub C. O., Arner P. (2012). Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes 61, 1986–1993 10.2337/db11-1508 - DOI - PMC - PubMed
-
- Baker M., Gaukrodger N., Mayosi B. M., Imrie H., Farrall M., Watkins H., Connell J. M., Avery P. J., Keavney B. (2005). Association between common polymorphisms of the proopiomelanocortin gene and body fat distribution: a family study. Diabetes 54, 2492–2496 - PubMed
-
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. (1990). The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell 61, 49–59 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

