Engineering microbial chemical factories to produce renewable "biomonomers"
- PMID: 22969753
- PMCID: PMC3430982
- DOI: 10.3389/fmicb.2012.00313
Engineering microbial chemical factories to produce renewable "biomonomers"
Abstract
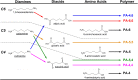
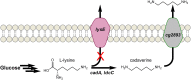
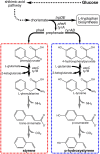
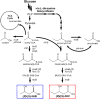
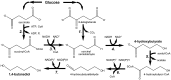
By applying metabolic engineering tools and strategies to engineer synthetic enzyme pathways, the number and diversity of commodity and specialty chemicals that can be derived directly from renewable feedstocks is rapidly and continually expanding. This of course includes a number of monomer building-block chemicals that can be used to produce replacements to many conventional plastic materials. This review aims to highlight numerous recent and important advancements in the microbial production of these so-called "biomonomers." Relative to naturally-occurring renewable bioplastics, biomonomers offer several important advantages, including improved control over the final polymer structure and purity, the ability to synthesize non-natural copolymers, and allowing products to be excreted from cells which ultimately streamlines downstream recovery and purification. To highlight these features, a handful of biomonomers have been selected as illustrative examples of recent works, including polyamide monomers, styrenic vinyls, hydroxyacids, and diols. Where appropriate, examples of their industrial penetration to date and end-product uses are also highlighted. Novel biomonomers such as these are ultimately paving the way toward new classes of renewable bioplastics that possess a broader diversity of properties than ever before possible.
Keywords: bioplastics; biopolymers; metabolic engineering; monomers.
Figures

Similar articles
-
Microbial Production of Biodegradable Lactate-Based Polymers and Oligomeric Building Blocks From Renewable and Waste Resources.Front Bioeng Biotechnol. 2021 Feb 4;8:618077. doi: 10.3389/fbioe.2020.618077. eCollection 2020. Front Bioeng Biotechnol. 2021. PMID: 33614605 Free PMC article. Review.
-
Creating pathways towards aromatic building blocks and fine chemicals.Curr Opin Biotechnol. 2015 Dec;36:1-7. doi: 10.1016/j.copbio.2015.07.004. Epub 2015 Aug 8. Curr Opin Biotechnol. 2015. PMID: 26264997 Review.
-
Microbial conversion of biomass into bio-based polymers.Bioresour Technol. 2017 Dec;245(Pt B):1664-1673. doi: 10.1016/j.biortech.2017.06.135. Epub 2017 Jun 27. Bioresour Technol. 2017. PMID: 28688739 Review.
-
New pathways and metabolic engineering strategies for microbial synthesis of diols.Curr Opin Biotechnol. 2022 Dec;78:102845. doi: 10.1016/j.copbio.2022.102845. Epub 2022 Nov 17. Curr Opin Biotechnol. 2022. PMID: 36403537 Review.
-
Recent advances in lignocellulosic biomass white biotechnology for bioplastics.Bioresour Technol. 2022 Jan;344(Pt B):126165. doi: 10.1016/j.biortech.2021.126165. Epub 2021 Oct 23. Bioresour Technol. 2022. PMID: 34695585 Review.
Cited by
-
Using Genome-scale Models to Predict Biological Capabilities.Cell. 2015 May 21;161(5):971-987. doi: 10.1016/j.cell.2015.05.019. Cell. 2015. PMID: 26000478 Free PMC article. Review.
-
Synthetic biology strategies for improving microbial synthesis of "green" biopolymers.J Biol Chem. 2018 Apr 6;293(14):5053-5061. doi: 10.1074/jbc.TM117.000368. Epub 2018 Jan 16. J Biol Chem. 2018. PMID: 29339554 Free PMC article. Review.
-
Expanding beyond canonical metabolism: Interfacing alternative elements, synthetic biology, and metabolic engineering.Synth Syst Biotechnol. 2017 Dec 19;3(1):20-33. doi: 10.1016/j.synbio.2017.12.002. eCollection 2018 Mar. Synth Syst Biotechnol. 2017. PMID: 29911196 Free PMC article. Review.
-
Metabolic engineering of Escherichia coli for polyamides monomer δ-valerolactam production from feedstock lysine.Appl Microbiol Biotechnol. 2020 Dec;104(23):9965-9977. doi: 10.1007/s00253-020-10939-8. Epub 2020 Oct 16. Appl Microbiol Biotechnol. 2020. PMID: 33064187
-
Artificial de novo biosynthesis of hydroxystyrene derivatives in a tyrosine overproducing Escherichia coli strain.Microb Cell Fact. 2015 Jun 10;14:78. doi: 10.1186/s12934-015-0268-7. Microb Cell Fact. 2015. PMID: 26055892 Free PMC article.
References
-
- Bechthold I., Bretz K., Kabasci S., Kopitzky R., Springer A. (2008). Succinic acid: a new platform chemical for biobased polymers from renewable resources. Chem. Eng. Technol. 31, 647–654
-
- Bermudez M., Leon S., Aleman C., Munoz-Guerra S. (2000). Comparison of lamellar crystal structure and morphology of nylon 46 and nylon 5. Polymer 41, 8961–8973
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

