Synergistic Cytotoxic Effects of Ganoderma lucidum and Bacillus Calmette Guérin on Premalignant Urothelial HUC-PC Cells and Its Regulation on Proinflammatory Cytokine Secretion
- PMID: 22969822
- PMCID: PMC3434421
- DOI: 10.1155/2012/147896
Synergistic Cytotoxic Effects of Ganoderma lucidum and Bacillus Calmette Guérin on Premalignant Urothelial HUC-PC Cells and Its Regulation on Proinflammatory Cytokine Secretion
Abstract
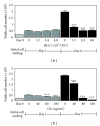
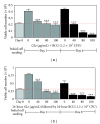
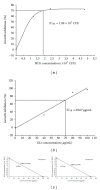
Bacillus Calmette-Guérin (BCG) is conventionally used as an adjuvant immunotherapy to reduce the recurrence of bladder cancer. To address the issues of efficacy and safety, an ethanol extract of Ganoderma lucidum (GLe) was evaluated for its interaction with BCG. In a model of premalignant human uroepithelial cells (HUC-PC), GLe exerted immediate cytotoxic effects while BCG showed a delayed response, given that both were immunological active in inducing the secretion of interleukin (IL)-6, IL-8, and monocyte chemotactic protein-1 (MCP-1). Synergistic cytotoxic effects were observed when cells were either coincubated with both drugs or firstly preincubated with GLe. Synergism between GLe and BCG was demonstrated to achieve a complete cytostasis in 24 hours, and such effects were progressed in the subsequent 5 days. However, the pretreatment of GLe resulted in suppression of IL-6, IL-8, and MCP-1 secretions without affecting the cytotoxicity. Given that numerous proinflammatory cytokines are associated with the high side effects toll of BCG, results herein suggested the potential implications of GL to supplement the BCG immunotherapy in bladder cancer, for better efficacy and reducing side effects.
Figures

References
-
- Simon R, Eltze E, Schäfer KL, et al. Cytogenetic analysis of multifocal bladder cancer supports a monoclonal origin and intraepithelial spread of tumor cells. Cancer Research. 2001;61(1):355–362. - PubMed
-
- Sakai I, Miyake H, Harada KI, Hara I, Inoue TA, Fujisawa M. Analysis of factors predicting intravesical recurrence of superficial transitional cell carcinoma of the bladder without concomitant carcinoma in situ. International Journal of Urology. 2006;13(11):1389–1392. - PubMed
-
- Patard JJ, Saint F, Velotti F, Abbou CC, Chopin DK. Immune response following intravesical bacillus Calmette-Guérin instillations in superficial madder cancer: a review. Urological Research. 1998;26(3):155–159. - PubMed
-
- Ludwig AT, Moore JM, Luo Y, et al. Tumor necrosis factor-related apoptosis-inducing ligand: a novel mechanism for bacillus Calmette-Guérin-induced antitumor activity. Cancer Research. 2004;64(10):3386–3390. - PubMed
-
- Paterson DL, Patel A. Bacillus Calmette-Guérin (BCG) immunotherapy for bladder cancer: review of complications and their treatment. Australian and New Zealand Journal of Surgery. 1998;68(5):340–344. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

