Activation of Spinal α2-Adrenoceptors Using Diluted Bee Venom Stimulation Reduces Cold Allodynia in Neuropathic Pain Rats
- PMID: 22969830
- PMCID: PMC3434467
- DOI: 10.1155/2012/784713
Activation of Spinal α2-Adrenoceptors Using Diluted Bee Venom Stimulation Reduces Cold Allodynia in Neuropathic Pain Rats
Abstract
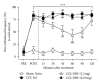
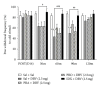
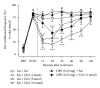
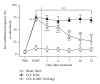
Cold allodynia is an important distinctive feature of neuropathic pain. The present study examined whether single or repetitive treatment of diluted bee venom (DBV) reduced cold allodynia in sciatic nerve chronic constriction injury (CCI) rats and whether these effects were mediated by spinal adrenergic receptors. Single injection of DBV (0.25 or 2.5 mg/kg) was performed into Zusanli acupoint 2 weeks post CCI, and repetitive DBV (0.25 mg/kg) was injected for 2 weeks beginning on day 15 after CCI surgery. Single treatment of DBV at a low dose (0.25 mg/kg) did not produce any anticold allodynic effect, while a high dose of DBV (2.5 mg/kg) significantly reduced cold allodynia. Moreover, this effect of high-dose DBV was completely blocked by intrathecal pretreatment of idazoxan (α2-adrenoceptor antagonist), but not prazosin (α1-adrenoceptor antagonist) or propranolol (nonselective β-adrenoceptor antagonist). In addition, coadministration of low-dose DBV (0.25 mg/kg) and intrathecal clonidine (α2-adrenoceptor agonist) synergically reduced cold allodynia. On the other hand, repetitive treatments of low-dose DBV showing no motor deficit remarkably suppressed cold allodynia from 7 days after DBV treatment. This effect was also reversed by intrathecal idazoxan injection. These findings demonstrated that single or repetitive stimulation of DBV could alleviate CCI-induced cold allodynia via activation of spinal α2-adrenoceptor.
Figures
References
-
- Zimmermann M. Pathobiology of neuropathic pain. European Journal of Pharmacology. 2001;429(1–3):23–37. - PubMed
-
- Martin TJ, Eisenach JC. Pharmacology of opioid and nonopioid analgesics in chronic pain states. Journal of Pharmacology and Experimental Therapeutics. 2001;299(3):811–817. - PubMed
-
- Namaka M, Gramlich CR, Ruhlen D, Melanson M, Sutton I, Major J. A treatment algorithm for neuropathic pain. Clinical Therapeutics. 2004;26(7):951–979. - PubMed
-
- Jensen TS, Gottrup H, Sindrup SH, Bach FW. The clinical picture of neuropathic pain. European Journal of Pharmacology. 2001;429(1–3):1–11. - PubMed
LinkOut - more resources
Full Text Sources

